Abstract
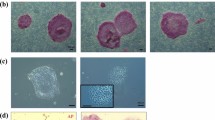
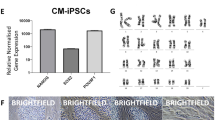
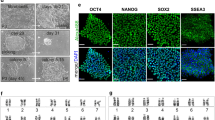
Derivation of embryonic stem (ES) cells genetically identical to a patient by somatic cell nuclear transfer (SCNT) holds the potential to cure or alleviate the symptoms of many degenerative diseases while circumventing concerns regarding rejection by the host immune system. However, the concept has only been achieved in the mouse, whereas inefficient reprogramming and poor embryonic development characterizes the results obtained in primates. Here, we used a modified SCNT approach to produce rhesus macaque blastocysts from adult skin fibroblasts, and successfully isolated two ES cell lines from these embryos. DNA analysis confirmed that nuclear DNA was identical to donor somatic cells and that mitochondrial DNA originated from oocytes. Both cell lines exhibited normal ES cell morphology, expressed key stem-cell markers, were transcriptionally similar to control ES cells and differentiated into multiple cell types in vitro and in vivo. Our results represent successful nuclear reprogramming of adult somatic cells into pluripotent ES cells and demonstrate proof-of-concept for therapeutic cloning in primates.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
McKay, R. Stem cells–hype and hope. Nature 406, 361–364 (2000)
Drukker, M. & Benvenisty, N. The immunogenicity of human embryonic stem-derived cells. Trends Biotechnol. 22, 136–141 (2004)
Takahashi, K. & Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 (2006); published online 10 August 2006
Okita, K., Ichisaka, T. & Yamanaka, S. Generation of germline-competent induced pluripotent stem cells. Nature 448, 313–317 (2007); published online 6 June 2007
Wernig, M. et al. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature 448, 318–324 (2007); published online 6 June 2007
Rideout, W. M., Hochedlinger, K., Kyba, M., Daley, G. Q. & Jaenisch, R. Correction of a genetic defect by nuclear transplantation and combined cell and gene therapy. Cell 109, 17–27 (2002)
Barberi, T. et al. Neural subtype specification of fertilization and nuclear transfer embryonic stem cells and application in parkinsonian mice. Nature Biotechnol. 21, 1200–1207 (2003)
Wilmut, I. et al. Somatic cell nuclear transfer. Nature 419, 583–586 (2002)
Mitalipov, S. M., Yeoman, R. R., Nusser, K. D. & Wolf, D. P. Rhesus monkey embryos produced by nuclear transfer from embryonic blastomeres or somatic cells. Biol. Reprod. 66, 1367–1373 (2002)
Simerly, C. et al. Molecular correlates of primate nuclear transfer failures. Science 300, 297 (2003)
Hall, V. J. et al. Developmental competence of human in vitro aged oocytes as host cells for nuclear transfer. Hum. Reprod. 22, 52–62 (2007)
Mitalipov, S. M. et al. Reprogramming following somatic cell nuclear transfer in primates is dependent upon nuclear remodeling. Hum. Reprod. 22, 2232–2242 (2007)
Birky, C. W. Uniparental inheritance of mitochondrial and chloroplast genes: mechanisms and evolution. Proc. Natl Acad. Sci. USA 92, 11331–11338 (1995)
Bowles, E. J., Campbell, K. H. & St John, J. C. Nuclear transfer: preservation of a nuclear genome at the expense of its associated mtDNA genome(s). Curr. Top. Dev. Biol. 77, 251–290 (2007)
Penedo, M. C. et al. Microsatellite typing of the rhesus macaque MHC region. Immunogenetics 57, 198–209 (2005)
Ferguson, B. et al. Single nucleotide polymorphisms (SNPs) distinguish Indian-origin and Chinese-origin rhesus macaques (Macaca mulatta). BMC Genomics 8, 43 (2007)
Mitalipov, S. et al. Isolation and characterization of novel rhesus monkey embryonic stem cell lines. Stem Cells 24, 2177–2186 (2006)
Byrne, J. A., Mitalipov, S. M., Clepper, L. & Wolf, D. P. Transcriptional profiling of rhesus monkey embryonic stem cells. Biol. Reprod. 75, 908–915 (2006)
Byrne, J. A., Clepper, L., Wolf, D. P. & Mitalipov, S. M. in International Society for Stem Cell Research, 5th Annual meeting 52 (Cairns, Queensland, Australia, 2007)
Simerly, C. et al. Embryogenesis and blastocyst development after somatic cell nuclear transfer in nonhuman primates: overcoming defects caused by meiotic spindle extraction. Dev. Biol. 276, 237–252 (2004)
Zhou, Q. et al. A comparative approach to somatic cell nuclear transfer in the rhesus monkey. Hum. Reprod. 21, 2564–2571 (2006)
Ng, S. C. et al. The first cell cycle after transfer of somatic cell nuclei in a non-human primate. Development 131, 2475–2484 (2004)
Yang, X. et al. Nuclear reprogramming of cloned embryos and its implications for therapeutic cloning. Nature Genet. 39, 295–302 (2007)
Munsie, M. J. et al. Isolation of pluripotent embryonic stem cells from reprogrammed adult mouse somatic cell nuclei. Curr. Biol. 10, 989–992 (2000)
Wakayama, T. et al. Differentiation of embryonic stem cell lines generated from adult somatic cells by nuclear transfer. Science 292, 740–743 (2001)
Hochedlinger, K. & Jaenisch, R. Monoclonal mice generated by nuclear transfer from mature B and T donor cells. Nature 415, 1035–1038 (2002)
Mombaerts, P. Therapeutic cloning in the mouse. Proc. Natl Acad. Sci. USA 100, 11924–11925 (2003); published online 29 August 2003
Wakayama, S. et al. Equivalency of nuclear transfer-derived embryonic stem cells to those derived from fertilized mouse blastocysts. Stem Cells 24, 2023–2033 (2006); published online 11 May 2006
Brambrink, T., Hochedlinger, K., Bell, G. & Jaenisch, R. ES cells derived from cloned and fertilized blastocysts are transcriptionally and functionally indistinguishable. Proc. Natl Acad. Sci. USA 103, 933–938 (2006); published online 17 January 2006
Lanza, R. P. et al. Generation of histocompatible tissues using nuclear transplantation. Nature Biotechnol. 20, 689–696 (2002); published online 3 June 2002
Martin, M. J. et al. Skin graft survival in genetically identical cloned pigs. Cloning Stem Cells 5, 117–121 (2003)
Yang, J. et al. Epigenetic marks in cloned rhesus monkey embryos: comparison with counterparts produced in vitro . Biol. Reprod. 76, 36–42 (2007)
Wakayama, S. et al. Establishment of mouse embryonic stem cell lines from somatic cell nuclei by nuclear transfer into aged, fertilization-failure mouse oocytes. Curr. Biol. 17, R120–R121 (2007)
Byrne, J., Mitalipov, S. & Wolf, D. Current progress with primate embryonic stem cells. Curr. Stem Cell Res. Ther. 1, 127–138 (2006)
Zelinski-Wooten, M. B., Hutchison, J. S., Hess, D. L., Wolf, D. P. & Stouffer, R. L. Follicle stimulating hormone alone supports follicle growth and oocyte development in gonadotrophin-releasing hormone antagonist-treated monkeys. Hum. Reprod. 10, 1658–1666 (1995)
Bavister, B. D. & Yanagimachi, R. The effects of sperm extracts and energy sources on the motility and acrosome reaction of hamster spermatozoa in vitro . Biol. Reprod. 16, 228–237 (1977)
McKiernan, S. H. & Bavister, B. D. Culture of one-cell hamster embryos with water soluble vitamins: pantothenate stimulates blastocyst production. Hum. Reprod. 15, 157–164 (2000)
Kuo, H. C. et al. Differentiation of monkey embryonic stem cells into neural lineages. Biol. Reprod. 68, 1727–1735 (2003)
Pearson, P. L. et al. Report of the committee on comparative mapping. Cytogenet. Cell Genet. 25, 82–95 (1979)
Rogers, J. et al. An initial genetic linkage map of the rhesus macaque (Macaca mulatta) genome using human microsatellite loci. Genomics 87, 30–38 (2006)
Domingo-Roura, X., Lopez-Giraldez, T., Shinohara, M. & Takenaka, O. Hypervariable microsatellite loci in the Japanese macaque (Macaca fuscata) conserved in related species. Am. J. Primatol. 43, 357–360 (1997)
St John, J. C. & Schatten, G. Paternal mitochondrial DNA transmission during nonhuman primate nuclear transfer. Genetics 167, 897–905 (2004)
Brazma, A. et al. Minimum information about a microarray experiment (MIAME)—toward standards for microarray data. Nature Genet. 29, 365–371 (2001)
Acknowledgements
The authors acknowledge the Division of Animal Resources and the Endocrine Services Cores at the Oregon National Primate Research Center for assistance and technical services. We thank M. Sparman, C. Ramsey and V. Dighe of the Assisted Reproductive Technology Core for their embryological and logistical assistance; J. Fanton and D. Jacobs for laparoscopic oocyte retrievals; B. Ferguson for performing the SNP analysis; C. Penedo for microsatellite analysis; and R. Stouffer, M. Grompe and R. Reijo Pera for reviewing this manuscript. Microarray assays were performed in the Affymetrix Microarray Core of the OHSU Gene Microarray Shared Resource. This study was supported by funds from ONPRC and NIH grants to S. Mitalipov, R. Stouffer and D. Dorsa.
Author Contributions S.M.M. and J.A.B. designed experiments, conducted SCNT and ES cell derivation. L.L.C. performed DNA/RNA isolations and stemness gene expression. J.A.B. analysed the microarray data and performed the mitochondrial DNA analysis. D.A.P. assisted with ES cell derivation and performed ES cell culture, characterization and differentiation. W.G.S. and M.N. performed the cytogenetic analysis. S.G. analysed teratomas. S.M.M., J.A.B. and D.P.W. analysed the data and wrote the paper.
Microarray data, including CEL and CHP files, and Supplementary Data files containing microarray analyses (Supplementary Data 3–7) have been deposited in the Gene Expression Omnibus (GEO) database with accession number GSE7748 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE7748).
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Information
The file contains Supplementary Figures 1-9 with Legends, Supplementary Tables 1-5 and the MIAME Checklist. (PDF 1085 kb)
Supplementary Video
The file contains Supplementary Video 1 showing contracting cardiomyocytes derived from differentiated CRES cell lines. (MOV 10738 kb)
Rights and permissions
About this article
Cite this article
Byrne, J., Pedersen, D., Clepper, L. et al. Producing primate embryonic stem cells by somatic cell nuclear transfer. Nature 450, 497–502 (2007). https://doi.org/10.1038/nature06357
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature06357
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.