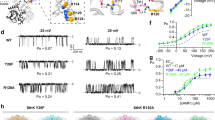
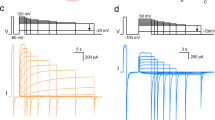
Abstract
Voltage-sensing domains enable membrane proteins to sense and react to changes in membrane voltage. Although identifiable S1–S4 voltage-sensing domains are found in an array of conventional ion channels and in other membrane proteins that lack pore domains, the extent to which their voltage-sensing mechanisms are conserved is unknown. Here we show that the voltage-sensor paddle, a motif composed of S3b and S4 helices, can drive channel opening with membrane depolarization when transplanted from an archaebacterial voltage-activated potassium channel (KvAP) or voltage-sensing domain proteins (Hv1 and Ci-VSP) into eukaryotic voltage-activated potassium channels. Tarantula toxins that partition into membranes can interact with these paddle motifs at the protein–lipid interface and similarly perturb voltage-sensor activation in both ion channels and proteins with a voltage-sensing domain. Our results show that paddle motifs are modular, that their functions are conserved in voltage sensors, and that they move in the relatively unconstrained environment of the lipid membrane. The widespread targeting of voltage-sensor paddles by toxins demonstrates that this modular structural motif is an important pharmacological target.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Jiang, Y. et al. X-ray structure of a voltage-dependent K+ channel. Nature 423, 33–41 (2003)
Kubo, Y., Baldwin, T. J., Jan, Y. N. & Jan, L. Y. Primary structure and functional expression of a mouse inward rectifier potassium channel. Nature 362, 127–133 (1993)
Li-Smerin, Y. & Swartz, K. J. Gating modifier toxins reveal a conserved structural motif in voltage-gated Ca2+ and K+ channels. Proc. Natl Acad. Sci. USA 95, 8585–8589 (1998)
Lu, Z., Klem, A. M. & Ramu, Y. Ion conduction pore is conserved among potassium channels. Nature 413, 809–813 (2001)
Murata, Y., Iwasaki, H., Sasaki, M., Inaba, K. & Okamura, Y. Phosphoinositide phosphatase activity coupled to an intrinsic voltage sensor. Nature 435, 1239–1243 (2005)
Long, S. B., Campbell, E. B. & Mackinnon, R. Voltage sensor of Kv1.2: structural basis of electromechanical coupling. Science 309, 903–908 (2005)
Long, S. B., Campbell, E. B. & Mackinnon, R. Crystal structure of a mammalian voltage-dependent Shaker family K+ channel. Science 309, 897–903 (2005)
Lee, S. Y., Lee, A., Chen, J. & Mackinnon, R. Structure of the KvAP voltage-dependent K+ channel and its dependence on the lipid membrane. Proc. Natl Acad. Sci. USA 102, 15441–15446 (2005)
Jiang, Y., Ruta, V., Chen, J., Lee, A. & MacKinnon, R. The principle of gating charge movement in a voltage-dependent K+channel. Nature 423, 42–48 (2003)
Tombola, F., Pathak, M. M. & Isacoff, E. Y. How far will you go to sense voltage? Neuron 48, 719–725 (2005)
Ahern, C. A. & Horn, R. Stirring up controversy with a voltage sensor paddle. Trends Neurosci. 27, 303–307 (2004)
Swartz, K. J. Towards a structural view of gating in potassium channels. Nature Rev. Neurosci. 5, 905–916 (2004)
Ruta, V., Chen, J. & MacKinnon, R. Calibrated measurement of gating-charge arginine displacement in the KvAP voltage-dependent K+ channel. Cell 123, 463–475 (2005)
Lee, S. Y. & MacKinnon, R. A membrane-access mechanism of ion channel inhibition by voltage sensor toxins from spider venom. Nature 430, 232–235 (2004)
Schmidt, D., Jiang, Q. X. & MacKinnon, R. Phospholipids and the origin of cationic gating charges in voltage sensors. Nature 444, 775–779 (2006)
Cuello, L. G., Cortes, D. M. & Perozo, E. Molecular architecture of the KvAP voltage-dependent K+ channel in a lipid bilayer. Science 306, 491–495 (2004)
Ahern, C. A. & Horn, R. Specificity of charge-carrying residues in the voltage sensor of potassium channels. J. Gen. Physiol. 123, 205–216 (2004)
Campos, F. V., Chanda, B., Roux, B. & Bezanilla, F. Two atomic constraints unambiguously position the S4 segment relative to S1 and S2 segments in the closed state of Shaker K channel. Proc. Natl Acad. Sci. USA 104, 7904–7909 (2007)
Grabe, M., Lai, H. C., Jain, M., Nung Jan, Y. & Yeh Jan, L. Structure prediction for the down state of a potassium channel voltage sensor. Nature 445, 550–553 (2007)
Tombola, F., Pathak, M. M., Gorostiza, P. & Isacoff, E. Y. The twisted ion-permeation pathway of a resting voltage-sensing domain. Nature 445, 546–549 (2007)
Chanda, B., Asamoah, O. K., Blunck, R., Roux, B. & Bezanilla, F. Gating charge displacement in voltage-gated ion channels involves limited transmembrane movement. Nature 436, 852–856 (2005)
Sasaki, M., Takagi, M. & Okamura, Y. A voltage sensor-domain protein is a voltage-gated proton channel. Science 312, 589–592 (2006)
Ramsey, I. S., Moran, M. M., Chong, J. A. & Clapham, D. E. A voltage-gated proton-selective channel lacking the pore domain. Nature 440, 1213–1216 (2006)
Ruta, V., Jiang, Y., Lee, A., Chen, J. & MacKinnon, R. Functional analysis of an archaebacterial voltage-dependent K+ channel. Nature 422, 180–185 (2003)
Frech, G. C., VanDongen, A. M., Schuster, G., Brown, A. M. & Joho, R. H. A novel potassium channel with delayed rectifier properties isolated from rat brain by expression cloning. Nature 340, 642–645 (1989)
Lu, Z., Klem, A. M. & Ramu, Y. Coupling between voltage sensors and activation gate in voltage-gated K+ channels. J. Gen. Physiol. 120, 663–676 (2002)
Aggarwal, S. K. & MacKinnon, R. Contribution of the S4 segment to gating charge in the Shaker K+ channel. Neuron 16, 1169–1177 (1996)
Seoh, S. A., Sigg, D., Papazian, D. M. & Bezanilla, F. Voltage-sensing residues in the S2 and S4 segments of the Shaker K+ channel. Neuron 16, 1159–1167 (1996)
Tempel, B. L., Papazian, D. M., Schwarz, T. L., Jan, Y. N. & Jan, L. Y. Sequence of a probable potassium channel component encoded at Shaker locus of Drosophila . Science 237, 770–775 (1987)
Soler-Llavina, G. J., Chang, T. H. & Swartz, K. J. Functional interactions at the interface between voltage-sensing and pore domains in the Shaker K(v) channel. Neuron 52, 623–634 (2006)
Swartz, K. J. & MacKinnon, R. Hanatoxin modifies the gating of a voltage-dependent K+ channel through multiple binding sites. Neuron 18, 665–673 (1997)
Swartz, K. J. & MacKinnon, R. Mapping the receptor site for hanatoxin, a gating modifier of voltage-dependent K+ channels. Neuron 18, 675–682 (1997)
Li-Smerin, Y. & Swartz, K. J. Localization and molecular determinants of the hanatoxin receptors on the voltage-sensing domain of a K+ channel. J. Gen. Physiol. 115, 673–684 (2000)
Li-Smerin, Y. & Swartz, K. J. Helical structure of the COOH terminus of S3 and its contribution to the gating modifier toxin receptor in voltage-gated ion channels. J. Gen. Physiol. 117, 205–218 (2001)
Lee, H. C., Wang, J. M. & Swartz, K. J. Interaction between extracellular hanatoxin and the resting conformation of the voltage-sensor paddle in Kv channels. Neuron 40, 527–536 (2003)
Phillips, L. R. et al. Voltage-sensor activation with a tarantula toxin as cargo. Nature 436, 857–860 (2005)
Swartz, K. J. Tarantula toxins interacting with voltage sensors in potassium channels. Toxicon 49, 213–230 (2007)
Ruta, V. & MacKinnon, R. Localization of the voltage-sensor toxin receptor on KvAP. Biochemistry 43, 10071–10079 (2004)
Jung, H. J. et al. Solution structure and lipid membrane partitioning of VSTx1, an inhibitor of the KvAP potassium channel. Biochemistry 44, 6015–6023 (2005)
Milescu, M. et al. Tarantula toxins interact with voltage sensors within lipid membranes. J. Gen. Physiol. 130, 497–511 (2007)
Lee, A. G. Lipid–protein interactions in biological membranes: a structural perspective. Biochim. Biophys. Acta 1612, 1–40 (2003)
Lampe, R. A. et al. Isolation and pharmacological characterization of omega-grammotoxin SIA, a novel peptide inhibitor of neuronal voltage-sensitive calcium channel responses. Mol. Pharmacol. 44, 451–460 (1993)
Lee, C. W. et al. Solution structure and functional characterization of SGTx1, a modifier of Kv2.1 channel gating. Biochemistry 43, 890–897 (2004)
Herrington, J. et al. Blockers of the delayed-rectifier potassium current in pancreatic β-cells enhance glucose-dependent insulin secretion. Diabetes 55, 1034–1042 (2006)
Papazian, D. M. et al. Electrostatic interactions of S4 voltage sensor in Shaker K+ channel. Neuron 14, 1293–1301 (1995)
Long, S. B., Tao, X., Campbell, E. B. & MacKinnon, R. Atomic structure of a voltage-dependent K+ channel in a lipid membrane-like environment. Nature doi:10.1038/nature06265 (this issue).
Siemens, J. et al. Spider toxins activate the capsaicin receptor to produce inflammatory pain. Nature 444, 208–212 (2006)
Swartz, K. J. & MacKinnon, R. An inhibitor of the Kv2.1 potassium channel isolated from the venom of a Chilean tarantula. Neuron 15, 941–949 (1995)
Garcia, M. L., Garcia-Calvo, M., Hidalgo, P., Lee, A. & MacKinnon, R. Purification and characterization of three inhibitors of voltage-dependent K+ channels from Leiurus quinquestriatus var. hebraeus venom. Biochemistry 33, 6834–6839 (1994)
Hoshi, T., Zagotta, W. N. & Aldrich, R. W. Biophysical and molecular mechanisms of Shaker potassium channel inactivation. Science 250, 533–538 (1990)
Acknowledgements
We thank F. Fontaine, M. Mayer, J. Mindell, S. Ramsey, S. Silberberg and members of the Swartz laboratory for discussions, and the NINDS DNA sequencing facility for DNA sequencing. We thank T. Kitaguchi for cloning KvAP and Y. Okamura for providing Ci-VSP complementary DNA. This work was supported by the Intramural Research Program of the NINDS, NIH. A.A.A. was partially supported by the NIH Undergraduate Scholarship Program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
The file contains Supplementary Figures 1-6 and Supplementary Tables 1-3 with Legends. (PDF 2774 kb)
Rights and permissions
About this article
Cite this article
Alabi, A., Bahamonde, M., Jung, H. et al. Portability of paddle motif function and pharmacology in voltage sensors. Nature 450, 370–375 (2007). https://doi.org/10.1038/nature06266
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature06266
This article is cited by
-
Therapeutic efficacy of voltage-gated sodium channel inhibitors in epilepsy
Acta Epileptologica (2023)
-
Role of the Voltage-Gated Proton Channel Hv1 in Nervous Systems
Neuroscience Bulletin (2023)
-
Inhibiting Hv1 channel in peripheral sensory neurons attenuates chronic inflammatory pain and opioid side effects
Cell Research (2022)
-
Insights on small molecule binding to the Hv1 proton channel from free energy calculations with molecular dynamics simulations
Scientific Reports (2020)
-
Global versus local mechanisms of temperature sensing in ion channels
Pflügers Archiv - European Journal of Physiology (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.