Abstract
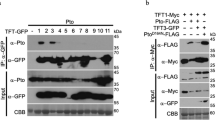
Pathogenic microbes use effectors to enhance susceptibility in host plants. However, plants have evolved a sophisticated immune system to detect these effectors using cognate disease resistance proteins1, a recognition that is highly specific, often elicits rapid and localized cell death, known as a hypersensitive response, and thus potentially limits pathogen growth2,3,4,5. Despite numerous genetic and biochemical studies on the interactions between pathogen effector proteins and plant resistance proteins, the structural bases for such interactions remain elusive. The direct interaction between the tomato protein kinase Pto and the Pseudomonas syringae effector protein AvrPto is known to trigger disease resistance and programmed cell death6,7 through the nucleotide-binding site/leucine-rich repeat (NBS-LRR) class of disease resistance protein Prf8. Here we present the crystal structure of an AvrPto–Pto complex. Contrary to the widely held hypothesis that AvrPto activates Pto kinase activity, our structural and biochemical analyses demonstrated that AvrPto is an inhibitor of Pto kinase in vitro. The AvrPto–Pto interaction is mediated by the phosphorylation-stabilized P+1 loop and a second loop in Pto, both of which negatively regulate the Prf-mediated defences in the absence of AvrPto in tomato plants. Together, our results show that AvrPto derepresses host defences by interacting with the two defence-inhibition loops of Pto.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Flor, H. H. Current status of the gene-for-gene concept. Annu. Rev. Phytopathol. 9, 275–296 (1971)
Belkhadir, Y., Subramaniam, R. & Dangl, J. L. Plant disease resistance protein signaling: NBS-LRR proteins and their partners. Curr. Opin. Plant Biol. 7, 391–399 (2004)
Chisholm, S. T., Coaker, G., Day, B. & Staskawicz, B. J. Host–microbe interactions: shaping the evolution of the plant immune response. Cell 124, 803–814 (2006)
Innes, R. W. Guarding the goods. New insights into the central alarm system of plants. Plant Physiol. 135, 695–701 (2004)
Pedley, K. F. & Martin, G. B. Molecular basis of Pto-mediated resistance to bacterial speck disease in tomato. Annu. Rev. Phytopathol. 41, 215–243 (2003)
Scofield, S. R. et al. Molecular basis of gene-for-gene specificity in bacterial speck disease of tomato. Science 274, 2063–2065 (1996)
Tang, X. et al. Initiation of plant disease resistance by physical interaction of AvrPto and Pto kinase. Science 274, 2060–2063 (1996)
Salmeron, J. M. et al. Tomato Prf is a member of the leucine-rich repeat class of plant disease resistance genes and lies embedded within the Pto kinase gene cluster. Cell 86, 123–133 (1996)
He, S. Y., Nomura, K. & Whittam, T. Type III secretion in mammalian and plant pathogens. Biochim. Biophys. Acta 1694, 181–206 (2004)
Galan, J. E. Salmonella interactions with host cells: type III secretion at work. Annu. Rev. Cell Dev. Biol. 17, 53–86 (2001)
Hueck, C. J. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol. Mol. Biol. Rev. 62, 379–433 (1998)
Kim, Y. J., Lin, N. C. & Martin, G. B. Two distinct Pseudomonas effector proteins interact with the Pto kinase and activate plant immunity. Cell 109, 589–598 (2002)
Mucyn, T. S. et al. The tomato NBARC-LRR protein Prf interacts with Pto kinase in vivo to regulate specific plant immunity. Plant Cell 18, 2792–2806 (2006)
Rathjen, J. P., Chang, J. H., Staskawicz, B. J. & Michelmore, R. W. Constitutively active Pto induces a Prf-dependent hypersensitive response in the absence of AvrPto. EMBO J. 18, 3232–3240 (1999)
Wu, A. J., Andriotis, V. M., Durrant, M. C. & Rathjen, J. P. A patch of surface-exposed residues mediates negative regulation of immune signaling by tomato Pto kinase. Plant Cell 16, 2809–2821 (2004)
He, P. et al. Specific bacterial suppressors of MAMP signaling upstream of MAPKKK in Arabidopsis innate immunity. Cell 125, 563–575 (2006)
Wulf, J., Pascuzzi, P. E., Fahmy, A., Martin, G. B. & Nicholson, L. K. The solution structure of type III effector protein AvrPto reveals conformational and dynamic features important for plant pathogenesis. Structure 12, 1257–1268 (2004)
Chang, J. H. et al. Functional analyses of the Pto resistance gene family in tomato and the identification of a minor resistance determinant in a susceptible haplotype. Mol. Plant Microbe Interact. 15, 281–291 (2002)
Frederick, R. D., Thilmony, R. L., Sessa, G. & Martin, G. B. Recognition specificity for the bacterial avirulence protein AvrPto is determined by Thr-204 in the activation loop of the tomato Pto kinase. Mol. Cell 2, 241–245 (1998)
Riely, B. K. & Martin, G. B. Ancient origin of pathogen recognition specificity conferred by the tomato disease resistance gene Pto. Proc. Natl Acad. Sci. USA 98, 2059–2064 (2001)
Huse, M. & Kuriyan, J. The conformational plasticity of protein kinases. Cell 109, 275–282 (2002)
Nolen, B., Taylor, S. & Ghosh, G. Regulation of protein kinases; controlling activity through activation segment conformation. Mol. Cell 15, 661–675 (2004)
Sessa, G., D'Ascenzo, M. & Martin, G. B. Thr38 and Ser198 are Pto autophosphorylation sites required for the AvrPto–Pto-mediated hypersensitive response. EMBO J. 19, 2257–2269 (2000)
Bossemeyer, D., Engh, R. A., Kinzel, V., Ponstingl, H. & Huber, R. Phosphotransferase and substrate binding mechanism of the cAMP-dependent protein kinase catalytic subunit from porcine heart as deduced from the 2.0 Å structure of the complex with Mn2+ adenylyl imidodiphosphate and inhibitor peptide PKI(5–24). EMBO J. 12, 849–859 (1993)
Stebbins, C. E. & Galan, J. E. Maintenance of an unfolded polypeptide by a cognate chaperone in bacterial type III secretion. Nature 414, 77–81 (2001)
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997)
Terwilliger, T. C. SOLVE and RESOLVE: automated structure solution and density modification. Methods Enzymol. 374, 22–37 (2003)
Jones, T. A., Zou, J. Y., Cowan, S. W. & Kjeldgaard, M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr. A 47, 110–119 (1997)
Murshudov, G. N., Vagin, A. A. & Dodson, E. J. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D53, 240–255 (1997)
Acknowledgements
We thank R. Innes, S. He and X. Tang for critical reading and comments on our manuscript, and Y. Dong and P. Liu at the BSRF (Beijing, China) beam line 3W1A for assistance with the data collection. We are grateful to X. Liu and L. Ma for help with SPR assay. This research is funded by a Chinese Ministry of Science and Technology grant to J.C. and to J.-M.Z.
Author Contributions W.X. purified, crystallized and determined the structure and performed biochemical assays; Y.Z. performed Agrobacterium-mediated transient expression; Q.L., Q. Huang and Q. Hao determined structure; J.L. and X.L. purified proteins; S.C. performed the mass spectrometry assay; J.-W.W. measured the half-maximal inhibitory concentration; R.B. and L.Z. were involved in the study design; and J.-M.Z. and J.C. designed the study, analysed data and prepared the manuscript.
The atomic coordinates and structure factors of the AvrPto–Pto complex have been deposited in the RCSB Protein Data Bank under accession code 2QKW.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Reprints and permissions information is available at www.nature.com/reprints. The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-14 and Legends, Supplementary Methods and additional references. (PDF 6504 kb)
Rights and permissions
About this article
Cite this article
Xing, W., Zou, Y., Liu, Q. et al. The structural basis for activation of plant immunity by bacterial effector protein AvrPto. Nature 449, 243–247 (2007). https://doi.org/10.1038/nature06109
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature06109
This article is cited by
-
Plant immunity research in China
Phytopathology Research (2023)
-
NOD-like receptor-mediated plant immunity: from structure to cell death
Nature Reviews Immunology (2021)
-
Advancement of research on plant NLRs evolution, biochemical activity, structural association, and engineering
Planta (2020)
-
Pattern recognition receptors and their interactions with bacterial type III effectors in plants
Genes & Genomics (2019)
-
CARK1 mediates ABA signaling by phosphorylation of ABA receptors
Cell Discovery (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.