Abstract
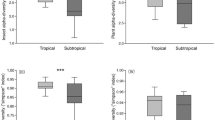
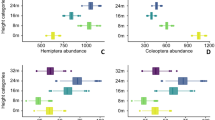
Recent advances in understanding insect communities in tropical forests1,2 have contributed little to our knowledge of large-scale patterns of insect diversity, because incomplete taxonomic knowledge of many tropical species hinders the mapping of their distribution records3. This impedes an understanding of global biodiversity patterns and explains why tropical insects are under-represented in conservation biology. Our study of approximately 500 species from three herbivorous guilds feeding on foliage (caterpillars, Lepidoptera), wood (ambrosia beetles, Coleoptera) and fruit (fruitflies, Diptera) found a low rate of change in species composition (beta diversity) across 75,000 square kilometres of contiguous lowland rainforest in Papua New Guinea, as most species were widely distributed. For caterpillars feeding on large plant genera, most species fed on multiple host species, so that even locally restricted plant species did not support endemic herbivores. Large plant genera represented a continuously distributed resource easily colonized by moths and butterflies over hundreds of kilometres. Low beta diversity was also documented in groups with differing host specificity (fruitflies and ambrosia beetles), suggesting that dispersal limitation does not have a substantial role in shaping the distribution of insect species in New Guinea lowland rainforests. Similar patterns of low beta diversity can be expected in other tropical lowland rainforests, as they are typically situated in the extensive low basins of major tropical rivers similar to the Sepik–Ramu region of New Guinea studied here.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Novotny, V. & Basset, Y. Host specificity of insect herbivores in tropical forests. Proc. R. Soc. B 272, 1083–1090 (2005)
Lewinsohn, T. M., Novotny, V. & Basset, Y. Insects on plants: diversity of herbivore assemblages revisited. Annu. Rev. Ecol. Evol. Syst. 36, 597–620 (2005)
Novotny, V. & Weiblen, G. D. From communities to continents: beta-diversity of herbivorous insects. Annal. Zool. Fenn. 42, 463–475 (2005)
Novotny, V., Clarke, A. R., Drew, R. A. I., Balagawi, S. & Clifford, B. Host specialization and species richness of fruit flies (Diptera: Tephritidae) in a New Guinea rain forest. J. Trop. Ecol. 21, 67–77 (2005)
Gaston, K. J. & Gauld, I. D. How many species of pimplines (Hymenoptera: Ichneumonidae) are there in Costa Rica? J. Trop. Ecol. 9, 491–499 (1993)
Orr, A. G. & Haeuser, C. L. Temporal and spatial patterns of butterfly diversity in a lowland tropical rainforest. In Tropical Rainforest Research — Current Issues (eds Edwards, D. S., Booth W. E. & Choy, S.) 125–138 (Kluwer, Dordrecht, 1996)
Erwin, T. L. The biodiversity question: How many species of terrestrial arthropods are there? In Forest Canopies 2nd edn (eds Lowman, M. D. & Rinker, H. B.) 259–269 (Elsevier, Burlington, 2004)
Ruokolainen, K., Tuomisto, H., Vormisto, J. & Pitman, N. Two biases in estimating range sizes of Amazonian plant species. J. Trop. Ecol. 18, 935–942 (2002)
Ødegaard, F. Host specificity, alpha- and beta-diversity of phytophagous beetles in two tropical forests in Panama. Biodivers. Conserv. 15, 83–105 (2006)
Beck, J. & Chey, V. K. Beta-diversity of geometrid moths from northern Borneo: effects of habitat, time and space. J. Anim. Ecol. 76, 230–237 (2007)
Brown, K. S. Geologic, evolutionary, and ecological bases of the diversification of Neotropical butterflies. In Tropical Rainforests. Past, Present, and Future (eds Bermingham, E., Dick, C. W. & Moritz, C.) 166–201 (Univ. Chicago Press, Chicago, 2005)
Howard, P. C. et al. Complementarity and the use of indicator groups for reserve selection in Uganda. Nature 394, 472–474 (1998)
Miller, S. E., Novotny, V. & Basset, Y. Studies on New Guinea moths. 1. Introduction (Lepidoptera). Proc. Entomol. Soc. Wash. 105, 1035–1043 (2003)
Novotny, V. et al. Local species richness of leaf-chewing insects feeding on woody plants from one hectare of a lowland rainforest. Cons. Biol. 18, 227–237 (2004)
Condit, R. et al. Beta-diversity in tropical forest trees. Science 295, 666–669 (2002)
Hubbell, S. P. The Unified Neutral Theory of Biodiversity and Biogeography (Princeton Univ. Press, Princeton, New Jersey, 2001)
Gascon, C. et al. Riverine barriers and the geographic distribution of Amazonian species. Proc. Natl Acad. Sci. USA 97, 13672–13677 (2000)
Hall, J. P. W. & Harvey, D. J. The phylogeography of Amazonia revisited: New evidence from riodinid butterflies. Evolution 56, 1489–1497 (2002)
Rosenzweig, M. L. Species Diversity in Space and Time (Cambridge Univ. Press, Cambridge, 1995)
Koleff, P., Lennon, J. J. & Gaston, K. J. Are there latitudinal gradients in species turnover? Glob. Ecol. Biogeogr. 12, 483–498 (2003)
Höft, R. Plants of New Guinea and the Solomon Islands. Dictionary of the Genera and Families of Flowering Plants and Ferns, Handbook no. 13. (Wau Ecology Institute, Wau, 1992)
Novotny, V. et al. Response to comment on “Why are there so many species of herbivorous insects in tropical rainforests?”. Science 315, 1666 (2007)
Ødegaard, F. How many species of arthropods? Erwin’s estimate revised. Biol. J. Linn. Soc. 71, 583–597 (2000)
Novotny, V. et al. Low host specificity of herbivorous insects in a tropical forest. Nature 416, 841–844 (2002)
Stork, N. E. How many species are there? Biodiv.. Cons. 2, 215–232 (1993)
Novotny, V. et al. An altitudinal comparison of caterpillar (Lepidoptera) assemblages on Ficus trees in Papua New Guinea. J. Biogeogr. 32, 1303–1314 (2005)
Barthlott, W., Lauer, W. & Placke, A. Global distribution of species diversity in vascular plants: towards a world map of phytodiversity. Erdkunde 50, 317–327 (1996)
Mittermeier, R. A. et al. Wilderness and biodiversity conservation. Proc. Natl Acad. Sci. USA 100, 10309–10313 (2003)
Pitman, N. C. A., Terborgh, J., Silman, M. R. & Nuňez, V. P. Tree species distributions in an upper Amazonian forest. Ecology 80, 2651–2661 (1999)
Chao, A., Chazdon, R. L., Colwell, R. K. & Shen, T. J. A new statistical approach for assessing similarity of species composition with incidence and abundance data. Ecol. Lett. 8, 148–159 (2005)
Reiner, E. J. & Robbins, R. G. The Middle Sepik Plains, New Guinea: A physiographic study. Geogr. Rev. 54, 20–44 (1964)
Wurm, S. A. & Hattori, S. Language Atlas of the Pacific Area (Australian Academy of the Humanities, Canberra, 1981)
Novotny, V. & Drozd, P. Size distribution of conspecific populations: peoples of New Guinea. Proc. R. Soc. Lond. B 267, 947–952 (2000)
Paijmans, K. (ed.) New Guinea Vegetation (Australian National Univ. Press, Canberra, 1976)
Wood, A. W. The soils of New Guinea. In Biogeography and Ecology of New Guinea (ed. Gressitt, J. L.) 73–86 (W. Junk, The Hague, 1982)
Fletcher, B. S. Dacine fruit flies collected during the dry season in the lowland rainforest of Madang Province, Papua New Guinea (Diptera: Tephritidae). Aust. J. Entomol. 37, 315–318 (1998)
Basset, Y. et al. Conservation and biological monitoring of tropical forests: the role of parataxonomists. J. Appl. Ecol. 41, 163–174 (2004)
Novotny, V. et al. Predictably simple: communities of caterpillars (Lepidoptera) feeding on rainforest trees in Papua New Guinea. Proc. R. Soc. Lond.. B 269, 2337–2344 (2002)
Pigram, C. J. & Davies, H. L. Terranes and the accretion history of the New Guinea orogen. J. Austral. Geol. Geophys. 10, 193–211 (1987)
Davies, H. L., Perembo, R. C. B., Winn, R. D. & KenGemar, P. Terranes of the New Guinea orogen. In Proceedings of the Geology Exploration and Mining Conference, Madang (ed. Hancock, G.) 61–66 (Australasian Institute of Mining and Metallurgy, Melbourne, 1997)
Abbott, L. D. Neogene tectonic reconstruction of the Adelbert-Finisterre-New Britain collision, northern Papua New Guinea. J. S. E. Asian Earth Sci. 11, 33–51 (1995)
Swadling, P. Changing shorelines and cultural orientations in the Sepik-Ramu, Papua New Guinea: implications for Pacific prehistory. World Archaeol. 29, 1–14 (1997)
Nix, H. A. & Kalma, J. D. Climate as a dominant control in the biogeography of northern Australia and New Guinea. In Bridge and Barrier: the Natural and Cultural History of Torres Straight (ed. Walker, D.) 61–92 (Australian National University, Canberra, 1972)
Leps, J., Novotny, V. & Basset, Y. Habitat and successional status of plants in relation to the communities of their leaf-chewing herbivores in Papua New Guinea. J. Ecol. 89, 186–199 (2001)
Hebert, P. D., Penton, N. E. H., Burns, J. M., Janzen, D. H. & Hallwachs, W. Ten species in one: DNA barcoding reveals cryptic species in the neotropical skipper butterfly Astraptes fulgerator. Proc. Natl Acad. Sci. USA 101, 14812–14817 (2004)
Hulcr, J. et al. DNA barcoding confirms polyphagy in a generalist moth, Homona mermerodes (Lepidoptera: Tortricidae). Mol. Ecol. Notes 7, 549–557 (2007)
Beaver, R. A. Insect-fungus relationship in the bark and ambrosia beetles. In Insect-Fungus Interactions (eds Wilding, N., Collins, N. M., Hammond, P. M. & Webber, J. F.) 121–143 (Academic, London, 1989)
Mueller, U. G., Gerardo, N. M., Aanen, D. K., Six, D. L. & Schultz, T. R. The evolution of agriculture in insects. Annu. Rev. Ecol. Syst. 36, 563–595 (2005)
Beaver, R. A. Host specificity of temperate and tropical animals. Nature 281, 139–141 (1979)
Farrell, B. D. et al. The evolution of agriculture in beetles (Curculionidae: Scolytinae and Platypodinae). Evolution 55, 2011–2027 (2001)
Drew, R. A. I. The tropical fruit flies (Diptera: Tephritidae: Dacinae) of the Australasian and Oceanian regions. Mem. Queensl. Mus. 26, 1–521 (1989)
Clarke, A. R. et al. Distribution and biogeography of Bactrocera and Dacus species (Diptera: Tephritidae) in Papua New Guinea. Aust. J. Entomol. 43, 148–156 (2004)
Koleff, P., Gaston, K. J. & Lennon, J. J. Measuring beta diversity for presence and absence data. J. Anim. Ecol. 72, 367–382 (2003)
Colwell, R. K. EstimateS: Statistical Estimation of Species Richness and Shared Species from Samples. Version 7. 5 <http://purl.oclc.org/estimates> (2005)
Acknowledgements
We thank D. Bito, W. Boen, A. Borney, L. Cizek, G. Damag, A. Krasa, J. Kua, R. Kutil, R. Lilip, M. Manaono, M. Rimandai, S. Sau, G. Sosanika, D. Stancik, V. Iwam and D. Wal for technical assistance, and V. O. Becker, J. Brown, A. Cognato, L. Craven, K. Damas, C. Drew, J. D. Holloway, M. Horak, K. Maes, J. Miller, E. G. Munroe, M. Shaffer, A. M. Solis, D. Stancik, W. Takeuchi and K. Tuck for taxonomic assistance. More than 150 insect collectors contributed to the study. P. Herbert provided DNA barcodes; J. Leps advised on statistical analysis; R. Condit, H. L. Davies, O. Diserud, J. Chave, M. Heads, J. Hrcek, D. H. Janzen, O. T. Lewis, F. Ødegaard, D. Storch and C. O. Webb commented on the manuscript. This work was supported by the National Science Foundation (USA), Grant Agencies of the Czech Republic, Czech Academy of Sciences and Czech Ministry of Education, Darwin Initiative for the Survival of Species (UK), David and Lucile Packard Fellowship in Science and Engineering, the National Geographic Society (USA), and The International Centre for the Management of Pest Fruit Flies (Griffith University). We thank Papua New Guinean customary landowners for allowing us to work in their forests. We dedicate this work to the late Richard Kutil.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Reprints and permissions information is available at www.nature.com/reprints. The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Results, Supplementary Figures S1-S5 with Legends, Supplementary Tables S1-S3 and Supplementary Appendices S1-S4. (PDF 272 kb)
Rights and permissions
About this article
Cite this article
Novotny, V., Miller, S., Hulcr, J. et al. Low beta diversity of herbivorous insects in tropical forests. Nature 448, 692–695 (2007). https://doi.org/10.1038/nature06021
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature06021
This article is cited by
-
Tree communities and functional traits determine herbivore compositional turnover
Oecologia (2023)
-
Climate, host and geography shape insect and fungal communities of trees
Scientific Reports (2023)
-
The patterns of co-occurrence variation are explained by the low dependence of bark beetles (Coleoptera: Scolytinae and Platypodinae) on hosts along altitude gradients
Frontiers in Zoology (2022)
-
Worldwide diversity of endophytic fungi and insects associated with dormant tree twigs
Scientific Data (2022)
-
Beta diversity of plant community and soil mesofauna along an elevational gradient in a mountainous semi-arid oak forest
Community Ecology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.