Abstract
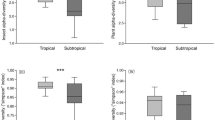
For numerous taxa, species richness is much higher in tropical than in temperate zone habitats1. A major challenge in community ecology and evolutionary biogeography is to reveal the mechanisms underlying these differences. For herbivorous insects, one such mechanism leading to an increased number of species in a given locale could be increased ecological specialization, resulting in a greater proportion of insect species occupying narrow niches within a community. We tested this hypothesis by comparing host specialization in larval Lepidoptera (moths and butterflies) at eight different New World forest sites ranging in latitude from 15° S to 55° N. Here we show that larval diets of tropical Lepidoptera are more specialized than those of their temperate forest counterparts: tropical species on average feed on fewer plant species, genera and families than do temperate caterpillars. This result holds true whether calculated per lepidopteran family or for a caterpillar assemblage as a whole. As a result, there is greater turnover in caterpillar species composition (greater β diversity) between tree species in tropical faunas than in temperate faunas. We suggest that greater specialization in tropical faunas is the result of differences in trophic interactions; for example, there are more distinct plant secondary chemical profiles from one tree species to the next in tropical forests than in temperate forests as well as more diverse and chronic pressures from natural enemy communities.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Hillebrand, H. On the generality of the latitudinal diversity gradient. Am. Nat. 163, 192–211 (2004)
Hutchinson, G. E. Homage to Santa Rosalia, or Why are there so many kinds of animals? Am. Nat. 93, 145–159 (1959)
MacArthur, R. H. & Levins, R. The limiting similarity, convergence, and divergence of coexisting species. Am. Nat. 101, 377–385 (1967)
Connell, J. H. Diversity in tropical rain forests and coral reefs. Science 199, 1302–1310 (1978)
Grant, P. R. & Grant, B. R. Evolution of character displacement in Darwin’s finches. Science 313, 224–226 (2006)
Farrell, B. D. ‘Inordinate fondness’ explained: why are there so many beetles? Science 281, 555–559 (1998)
Schluter, D. The Ecology of Adaptive Radiation (Oxford Univ. Press, Oxford, 2000)
Thompson, J. N. The Geographic Mosaic of Coevolution (Univ. of Chicago Press, Chicago, 2005)
Darwin, C. On the Origin of Species by Means of Natural Selection, or the Preservation of Favoured Races in the Struggle for Life (John Murray, London, 1859)
Wallace, A. Tropical Nature and Other Essays (MacMillan, London, 1878)
Armbruster, S. W. in Plant–Pollinator Interactions (eds Wasser, N. M. & Ollerton, J.) 260–282 (Chicago Univ. Press, Chicago, 2006)
Novotny, V. et al. Why are there so many species of herbivorous insects in tropical rainforests? Science 313, 1115–1118 (2006)
Price, P. W. in Plant–Animal Interactions (eds Herrera, C. M. & Pellmyr, O.) 3–26 (Blackwell, Oxford, 2002)
Thomas, C. D. Fewer species. Nature 347, 237 (1990)
Lewinsohn, T. M., Novotny, V. & Basset, Y. Insects on plants: Diversity of herbivore assemblages revisited. Annu. Rev. Ecol. Syst. 36, 597–620 (2005)
Irschick, D., Dyer, L. A. & Sherry, T. Phylogenetic methods for studying specialization. Oikos 110, 404–408 (2005)
Ødegaard, F., Diserud, O. H. & Østbye, K. The importance of plant relatedness for host utilization among phytophagous insects. Ecol. Lett. 8, 612–617 (2005)
Hajibabaei, M., Janzen, D. H., Burns, J. M., Hallwachs, W. & Hebert, P. D. N. DNA barcodes distinguish species of tropical Lepidoptera. Proc. Natl Acad. Sci. USA 103, 968–971 (2006)
Janzen, D. H. Coevolution of mutualism between ants and acacias in Central America. Evolution Int. J. Org. Evolution 20, 249–275 (1966)
Coley, P. D. & Barone, J. A. Herbivory and plant defenses in tropical forests. Annu. Rev. Ecol. Syst. 27, 305–335 (1996)
Dyer, L. A. & Coley, P. D. in Multitrophic Level Interactions (eds Tscharntke, T. & Hawkins, B.) 67–88 (Cambridge Univ. Press, Cambridge, 2002)
Marquis, R. J. & Braker, H. E. in La Selva; Ecology and Natural History of a Tropical Rain Forest (eds McDade, L., Bawa K. L., Hespenheide, H. A. & Hartshorn, G. S.) 261–281 (Univ. of Chicago Press, Chicago, 1994)
Wilf, P., Labandeira, C. C., Johnson, K. R. & Ellis, B. Decoupled plant and insect diversity after the end-Cretaceous extinction. Science 313, 1112–1115 (2006)
Hawkins, B. A. & Porter, E. E. Does herbivore diversity depend on plant diversity? The case of California butterflies. Am. Nat. 161, 40–49 (2003)
Tilman, D. Resource Competition and Community Structure (Princeton Univ. Press, Princeton, 1982)
Chase, J. M. & Leibold, M. A. Ecological Niches: Linking Classical and Contemporary Approaches (Univ. of Chicago Press, Chicago, 2003)
Colwell, R. K. & Coddington, J. A. Estimating terrestrial biodiversity through extrapolation. Phil. Trans. R. Soc. Lond. B 345, 101–118 (1994)
Kiflawi, M. & Spencer, M. Confidence intervals and hypothesis testing for beta diversity. Ecology 85, 2895–2900 (2004)
Whittaker, R. Vegetation of the Siskiyou Mountains, Oregon and California. Ecol. Monogr. 30, 279–338 (1960)
McGugan, B. M. Forest Lepidoptera of Canada Recorded by the Forest Insect Survey Vol. 1, Papilionidae to Arctiidae (Department of Forestry of Canada, Ottawa, 1958)
Gentry, G. & Dyer, L. A. On the conditional nature of neotropical caterpillar defenses against their natural enemies. Ecology 83, 3108–3119 (2002)
Stireman, J. O. & Singer, M. S. Determinants of parasitoid–host associations: insights from a natural tachinid–lepidopteran community. Ecology 84, 296–310 (2003)
Stireman, J. O. et al. Climatic unpredictability and caterpillar parasitism: implications of global warming. Proc. Natl Acad. Sci. USA 102, 17384–17387 (2005)
Coley, P. D., Bateman, M. L. & Kursar, T. A. The effects of plant quality on caterpillar growth and defense against natural enemies. Oikos 115, 219–228 (2006)
Diniz, I. R. & Morais, H. C. Lepidopteran caterpillar fauna of cerrado host plants. Biodiv. Conserv. 6, 817–836 (1997)
Acknowledgements
We thank D. Janzen, D. Gruner, G. Rodriguez, J. Landosky and R. Forkner for helpful suggestions for improving the manuscript; G. Howse for making available the CFIS data; E. Selig, A. Frevert, J. McGrath and M. Walker for their efforts in constructing the Canadian database; and a large number of taxonomists, field assistants and students for their help in generating all data. Funding came from the US National Science Foundation, Earthwatch Institute, National Geographic, Tulane University, University of Missouri Research Award, Wesleyan University’s Hughes Summer Research Program, and the National Institute for Climate Change Research.
Author Contributions All authors designed and performed data collection protocols and contributed substantially to writing the paper; M.S.S. proposed the original idea for the paper; M.S.S. and L.A.D. designed the analyses and wrote the first full draft of the paper; J.O.S., R. J. Marquis, J. T. Lill and R. E. Ricklefs contributed extensive revisions; L.A.D. performed statistical analyses and created figures.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Reprints and permissions information is available at www.nature.com/reprints. The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Data, Supplementary Methods and Supplementary Figures S1-S2 with Legends. (PDF 365 kb)
Rights and permissions
About this article
Cite this article
Dyer, L., Singer, M., Lill, J. et al. Host specificity of Lepidoptera in tropical and temperate forests. Nature 448, 696–699 (2007). https://doi.org/10.1038/nature05884
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature05884
This article is cited by
-
Tree communities and functional traits determine herbivore compositional turnover
Oecologia (2023)
-
Ecological drivers of bee cognition: insights from stingless bees
Behavioral Ecology and Sociobiology (2023)
-
The patterns of co-occurrence variation are explained by the low dependence of bark beetles (Coleoptera: Scolytinae and Platypodinae) on hosts along altitude gradients
Frontiers in Zoology (2022)
-
A comparative study on insect longevity: tropical moths do not differ from their temperate relatives
Evolutionary Ecology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.