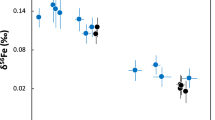
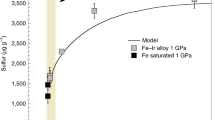
Abstract
Small isotopic differences between the silicate minerals in planets may have developed as a result of processes associated with core formation, or from evaporative losses during accretion as the planets were built up. Basalts from the Earth and the Moon do indeed appear to have iron isotopic compositions that are slightly heavy relative to those from Mars, Vesta and primitive undifferentiated meteorites1,2,3,4 (chondrites). Explanations for these differences have included evaporation during the ‘giant impact’ that created the Moon (when a Mars-sized body collided with the young Earth). However, lithium5 and magnesium6, lighter elements with comparable volatility7,8,9, reveal no such differences, rendering evaporation unlikely as an explanation. Here we show that the silicon isotopic compositions of basaltic rocks from the Earth and the Moon are also distinctly heavy. A likely cause is that silicon is one of the light elements in the Earth’s core. We show that both the direction and magnitude of the silicon isotopic effect are in accord with current theory10 based on the stiffness of bonding in metal and silicate. The similar isotopic composition of the bulk silicate Earth and the Moon is consistent with the recent proposal11 that there was large-scale isotopic equilibration during the giant impact. We conclude that Si was already incorporated as a light element in the Earth’s core before the Moon formed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Poitrasson, F., Halliday, A. N., Lee, D.-C., Levasseur, S. & Teutsch, N. Iron isotope differences between Earth, Moon, Mars and Vesta as possible records of contrasted accretion mechanisms. Earth Planet. Sci. Lett. 223, 253–266 (2004)
Weyer, S. et al. Iron isotope fractionation during planetary differentiation. Earth Planet. Sci. Lett. 240, 251–264 (2005)
Williams, H. M. et al. Iron isotope fractionation in iron meteorites: New insights into metal-sulfide segregation and planetary accretion. Earth Planet. Sci. Lett. 250, 486–500 (2006)
Schoenberg, R. & von Blanckenburg, F. Modes of planetary-scale Fe isotope fractionation. Earth Planet. Sci. Lett. 252, 342–359 (2006)
Magna, T., Wiechert, U. & Halliday, A. N. New constraints on the lithium isotope compositions of the Moon and terrestrial planets. Earth Planet. Sci. Lett. 243, 336–353 (2006)
Wiechert, U. & Halliday, A. N. Non-chondritic magnesium and the origins of the inner terrestrial planets. Earth Planet. Sci. Lett. 256, 360–371 (2007)
Lodders, K. Solar system abundances and condensation temperatures of the elements. Astrophys. J. 591, 1220–1247 (2003)
Wang, J., Davis, A. M., Clayton, R. N., Mayeda, T. K. & Hashimoto, A. Chemical and isotopic fractionation during the evaporation of the FeO-MgO-SiO2-CaOAl2O3-TiO2 rare earth element melt system. Geochim. Cosmochim. Acta 65, 479–494 (2001)
Clayton, R. N., Hinton, R. W. & Davis, A. M. Isotopic variations in the rock-forming elements in meteorites. Phil. Trans. R. Soc. Lond. A 325, 483–501 (1988)
Schauble, E. A. Applying stable isotope fractionation theory to new systems. Rev. Mineral. Geochem. 55, 65–111 (2004)
Pahlevan, K. & Stevenson, D. J. The oxygen isotope similarity between the Earth and Moon – source region or formation process? Lunar Planet. Sci. XXXVI, 2382 (2005)
Georg, R. B., Reynolds, B. C., Frank, M. & Halliday, A. N. New sample preparation techniques for the precise determination of the Si isotope composition of natural samples using MC-ICP-MS. Chem. Geol. 235, 95–104 (2006)
Clayton, R. N. Oxygen isotopes in meteorites. Annu. Rev. Earth Planet. Sci. 21, 115–149 (1993)
Douthitt, C. B. The geochemistry of the stable isotopes of silicon. Geochim. Cosmochim. Acta 46, 1449–1458 (1982)
Epstein, S. & Taylor, H. P. 18O/16O, 30Si/28Si, D/H and 13C/12C studies on lunar rocks and minerals. Science 167, 533–535 (1970)
Taylor, H. P. & Epstein, S. Oxygen and silicon isotope ratios of lunar rock 12013. Earth Planet. Sci. Lett. 9, 208–210 (1970)
Molini-Velsko, C., Mayeda, T. K. & Clayton, R. N. Isotopic composition of silicon in meteorites. Geochim. Cosmochim. Acta 50, 2719–2726 (1986)
Wade, J. & Wood, B. J. Core formation and the oxidation state of the Earth. Earth Planet. Sci. Lett. 236, 78–95 (2005)
Allègre, C. J., Poirier, J.-P., Humler, E. & Hofmann, A. W. The chemical composition of the Earth. Earth Planet. Sci. Lett. 134, 515–526 (1995)
Palme, H. & O’Neill, H. St C. in The Mantle (ed. Carlson, R.W.) Vol. 2, Treatise of Geochemistry (eds Holland, H. D. & Turekian, K. K.) 1–38 (Elsevier-Pergamon, Oxford, 2003)
Takafuji, N., Hirose, K., Mitome, M. & Bando, Y. Solubilities of O and Si in liquid iron in equilibrium with (Mg,Fe)SiO3 perovskite and the light elements in the core. Geophys. Res. Lett. 32 L06313 doi: 10.1029/2005GL022773 (2005)
Newsom, H. E. Accretion and core formation in the Earth: evidence from siderophile elements. In Origin of the Earth (eds Newsom, H. E. & Jones, J. H.) 273–288 (Oxford Univ. Press, Oxford, 1990)
Kieffer, S. W. Thermodynamics and lattice vibrations of minerals. 5. Application to phase equilibria, isotopic fractionation and high-pressure thermodynamic properties. Rev. Geophys. Space Phys. 20, 827–849 (1982)
Baroni, S., Gironcoli, S., Dal Corso, A. & Giannozzi, P. Phonons and related crystal properties from density-functional perturbation theory. Rev. Mod. Phys. 73, 515–562 (2001)
Gonze, X. et al. First-principles computation of material properties: the ABINIT software project. Comput. Mater. Sci. 25, 478–492 (2002)
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996)
Schauble, E. A., Ghosh, P. & Eiler, J. M. Preferential formation of 13C-18O bonds in carbonate minerals, estimated using first-principles lattice dynamics. Geochim. Cosmochim. Acta 70, 2510–2529 (2006)
Randl, O. G. et al. Lattice dynamics and related diffusion properties of intermetallics: I. Fe3Si. J. Phys. Condens. Matter 7, 5983–5999 (1995)
Williams, H. M. et al. Systematic iron isotope variations in mantle rocks and minerals: the effects of partial melting and oxygen fugacity. Earth Planet. Sci. Lett. 235, 435–452 (2005)
Wiechert, U. et al. Oxygen isotopes and the Moon-forming giant impact. Science 294, 345–348 (2001)
Acknowledgements
We are grateful to C. Smith and colleagues at the Natural History Museum, London, for aliquots of most of the meteorite samples and to CAPTEM for the lunar samples. MORB and Loihi basalt glasses were provided by M. Garcia, C. Langmuir and W. White. We also thank F. Oberli and colleagues for continuing technical assistance for the isotope facility at ETH Zurich, and SNF, PPARC (now STFC), the NSF and Oxford University for providing financial support. We thank S. Nielsen, H. Williams, D. Stevenson and K. Pahlevan for discussion. The manuscript was improved following comments from T. Elliott.
Author Contributions R.B.G. developed the isotopic methods, produced all of the analytical data and most of the tables and figures, and contributed to the modelling and interpretation. A.N.H. conceived the project, organised sample acquisition, contributed to the interpretation and modelling and wrote most of the text. E.A.S. developed the isotopic fractionation theory and associated calculations and interpretations and wrote important sections of the text. B.C.R. developed the isotopic methods with R.B.G. and made critical standard calibration measurements.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Reprints and permissions information is available at www.nature.com/reprints. The authors declare no competing financial interests.
Supplementary information
Supplementary Information 1
This file contains Supplementary Discussion on the calculation of the Fe isotopic fractionation expected from perovskite pumping and mantle self-oxidation and additional references. (PDF 71 kb)
Rights and permissions
About this article
Cite this article
Georg, R., Halliday, A., Schauble, E. et al. Silicon in the Earth’s core. Nature 447, 1102–1106 (2007). https://doi.org/10.1038/nature05927
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature05927
This article is cited by
-
Nonlinear Deformation of Fe, FeSi and FeCrSi with BCC Structure Under Pressure
Transactions of the Indian Institute of Metals (2023)
-
Accurate temperature diagnostics for matter under extreme conditions
Nature Communications (2022)
-
The accretion of planet Earth
Nature Reviews Earth & Environment (2022)
-
Early planetary processes and light elements in iron-dominated cores
Acta Geochimica (2022)
-
Light elements in the Earth’s core
Nature Reviews Earth & Environment (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.