Abstract
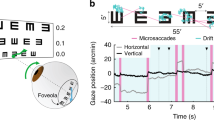
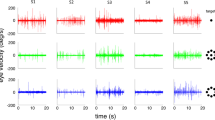
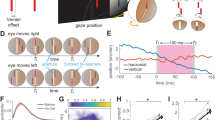
Our eyes are constantly in motion. Even during visual fixation, small eye movements continually jitter the location of gaze1,2,3,4. It is known that visual percepts tend to fade when retinal image motion is eliminated in the laboratory5,6,7,8,9. However, it has long been debated whether, during natural viewing, fixational eye movements have functions in addition to preventing the visual scene from fading10,11,12,13,14,15,16,17. In this study, we analysed the influence in humans of fixational eye movements on the discrimination of gratings masked by noise that has a power spectrum similar to that of natural images. Using a new method of retinal image stabilization18, we selectively eliminated the motion of the retinal image that normally occurs during the intersaccadic intervals of visual fixation. Here we show that fixational eye movements improve discrimination of high spatial frequency stimuli, but not of low spatial frequency stimuli. This improvement originates from the temporal modulations introduced by fixational eye movements in the visual input to the retina, which emphasize the high spatial frequency harmonics of the stimulus. In a natural visual world dominated by low spatial frequencies, fixational eye movements appear to constitute an effective sampling strategy by which the visual system enhances the processing of spatial detail.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ratliff, F. & Riggs, L. A. Involuntary motions of the eye during monocular fixation. J. Exp. Psychol. 40, 687–701 (1950)
Ditchburn, R. W. Eye movements in relation to retinal action. Opt. Acta 1, 171–176 (1955)
Steinman, R. M., Haddad, G. M., Skavenski, A. A. & Wyman, D. Miniature eye movement. Science 181, 810–819 (1973)
Murakami, I. & Cavanagh, P. A jitter after-effect reveals motion-based stabilization of vision. Nature 395, 798–801 (1998)
Ditchburn, R. W. & Ginsborg, B. L. Vision with a stabilized retinal image. Nature 170, 36–37 (1952)
Riggs, L. A. & Ratliff, F. The effects of counteracting the normal movements of the eye. J. Opt. Soc. Am. 42, 872–873 (1952)
Riggs, L. A., Ratliff, F., Cornsweet, J. C. & Cornsweet, T. N. The disappearance of steadily fixated visual test objects. J. Opt. Soc. Am. 43, 495–501 (1953)
Yarbus, A. L. Eye Movements and Vision (Plenum, New York, 1967)
Martinez-Conde, S., Macknik, S. L., Troncoso, X. G. & Dyar, T. A. Microsaccades counteract fading during fixation. Neuron 49, 297–305 (2006)
Hering, E. Über die Grenzen der Sehschärfe. Berichte der Königlichen Sächsischen Gesellshaft der Wissenschaften. Math. Phys. Klasse 20, 16–24 (1899)
Averill, H. I. & Weymouth, F. W. Visual perception and the retinal mosaic, II. The influence of eye movements on the displacement threshold. J. Comp. Psychol. 5, 147–176 (1925)
Marshall, W. H. & Talbot, S. A. in Biological Symposia——Visual Mechanisms. Vol. 7 (ed. Kluver, H.) 117–164 (Cattel, Lancaster, Pennsylvania, 1942)
Arend, L. E. Spatial differential and integral operations in human vision: implications of stabilized retinal image fading. Psychol. Rev. 80, 374–395 (1973)
Ahissar, E. & Arieli, A. Figuring space by time. Neuron 32, 185–201 (2001)
Greschner, M., Bongard, M., Rujan, P. & Ammermüller, J. Retinal ganglion cell synchronization by fixational eye movements improves feature estimation. Nature Neurosci. 5, 341–347 (2002)
Ölveczky, B. P., Baccus, S. A. & Meister, M. Segregation of object and background motion in the retina. Nature 423, 401–408 (2003)
Rucci, M. & Casile, A. Fixational instability and natural image statistics: implications for early visual representations. Network Comp. Neur. Syst. 16, 121–138 (2005)
Santini, F., Redner, G., Iovin, R. & Rucci, M. EyeRIS: A general-purpose system for eye movement contingent display control. Behav. Res. Methods (in the press)
Koenderink, J. J. Contrast enhancement and the negative afterimage. J. Opt. Soc. Am. A 62, 685–689 (1972)
Kelly, D. H. Motion and vision. I. Stabilized images of stationary gratings. J. Opt. Soc. Am. 69, 1266–1274 (1979)
Tulunay-Keesey, Ü. Fading of stabilized retinal images. J. Opt. Soc. Am. 72, 440–447 (1982)
Tulunay-Keesey, Ü. & Jones, R. M. The effect of micromovements of the eye and exposure duration on contrast sensitivity. Vision Res. 16, 481–488 (1976)
Steinman, R. M., Cunitz, R. J., Timberlake, G. T. & Herman, M. Voluntary control of microsaccades during maintained monocular fixation. Science 155, 1577–1579 (1967)
Steinman, R. M. & Collewijn, H. Binocular retinal image motion during active head rotation. Vision Res. 20, 415–429 (1980)
Kapoula, Z. A., Robinson, D. A. & Hain, T. C. Motion of the eye immediately after a saccade. Exp. Brain Res. 61, 386–394 (1986)
Field, D. J. Relations between the statistics of natural images and the response properties of cortical cells. J. Opt. Soc. Am. A 4, 2379–2394 (1987)
Leopold, D. A. & Logothetis, N. K. Microsaccades differentially modulate neural activity in the striate and extrastriate visual cortex. Exp. Brain Res. 123, 341–345 (1998)
Martinez-Conde, S., Macknik, S. L. & Hubel, D. H. Microsaccadic eye movements and firing of single cells in the striate cortex of macaque monkeys. Nature Neurosci. 3, 251–258 (2000)
Snodderly, D. M., Kagan, I. & Gur, M. Selective activation of visual cortex neurons by fixational eye movements: implications for neural coding. Vis. Neurosci. 18, 259–277 (2001)
Macmillan, N. A. & Creelman, C. D. Detection Theory—A User’s Guide 2nd edn (L.Erlbaum Associates, London, 2005)
Acknowledgements
We thank E. Ahissar, G. Desbordes, W. S. Geisler, K. J. Nielsen, E. L. Schwartz, D. M. Snodderly and J. D. Victor for help. This work was supported by grants from the National Institute of Health and the National Science Foundation to M.R.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Reprints and permissions information is available at www.nature.com/reprints. The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-2 with Legends, Supplementary Methods and Supplementary Video Legends. The two Supplementary Figures show contrast sensitivity functions measured with prolonged retinal stabilization and the spatial characteristics of recorded eye movements. The Supplementary Methods section provides further details about the retinal stabilization technique used in the experiments. (PDF 248 kb)
Supplementary Video
This file contains Supplementary Video 1 which is a reconstruction of the visual input to the retina during a trial of Experiment 1. For clarity, the stimulus has been enlarged by elimination of the Gaussian window. The modulations resulting from fixational eye movements enhance the high-frequency grating. See Supplementary Video Legend for detail. (MOV 88 kb)
Supplementary Video
This file contains Supplementary Video 2 which is a reconstruction of the visual input to the retina during a trial of Experiment 1. For clarity, the stimulus has been enlarged by elimination of the Gaussian window. The modulations resulting from fixational eye movements enhance the high-frequency grating. See Supplementary Video Legend for detail. (MOV 87 kb)
Supplementary Video
This file contains Supplementary Video 3 which is a reconstruction of the visual input to the retina during a trial of Experiment 2. For clarity, the stimulus has been enlarged by elimination of the Gaussian window. The modulations resulting from fixational eye movements attenuate the low-frequency grating. See Supplementary Video Legend for detail. (MOV 147 kb)
Supplementary Video
This file contains Supplementary Video 4 which is a reconstruction of the visual input to the retina during a trial of Experiment 2. For clarity, the stimulus has been enlarged by elimination of the Gaussian window. The modulations resulting from fixational eye movements attenuate the low-frequency grating. See Supplementary Video Legend for detail. (MOV 156 kb)
Supplementary Video
This file contains Supplementary Video 5 which is areconstruction of the visual input to the retina during a fixation on a natural image. The modulations resulting from fixational eye movements enhance high spatial frequencies and attenuate low spatial frequencies. See Supplementary Video Legend for detail. (MOV 119 kb)
Rights and permissions
About this article
Cite this article
Rucci, M., Iovin, R., Poletti, M. et al. Miniature eye movements enhance fine spatial detail. Nature 447, 852–855 (2007). https://doi.org/10.1038/nature05866
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature05866
This article is cited by
-
A dynamic sequence of visual processing initiated by gaze shifts
Nature Neuroscience (2023)
-
Peripheral targets attenuate miniature eye movements during fixation
Scientific Reports (2023)
-
Opposite forms of adaptation in mouse visual cortex are controlled by distinct inhibitory microcircuits
Nature Communications (2022)
-
V1-bypassing suppression leads to direction-specific microsaccade modulation in visual coding and perception
Nature Communications (2022)
-
Fixational drift is driven by diffusive dynamics in central neural circuitry
Nature Communications (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.