Abstract
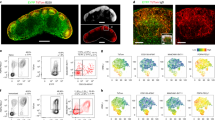
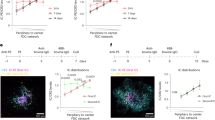
Germinal centres are specialized structures wherein B lymphocytes undergo clonal expansion, class switch recombination, antibody gene diversification and affinity maturation. Three to four antigen-specific B cells colonize a follicle to establish a germinal centre and become rapidly dividing germinal-centre centroblasts that give rise to dark zones1,2,3,4. Centroblasts produce non-proliferating centrocytes that are thought to migrate to the light zone of the germinal centre, which is rich in antigen-trapping follicular dendritic cells and CD4+ T cells5,6,7. It has been proposed that centrocytes are selected in the light zone on the basis of their ability to bind cognate antigen5,6,7,8. However, there have been no studies of germinal-centre dynamics or the migratory behaviour of germinal-centre cells in vivo. Here we report the direct visualization of B cells in lymph node germinal centres by two-photon laser-scanning microscopy in mice. Nearly all antigen-specific B cells participating in a germinal-centre reaction were motile and physically restricted to the germinal centre but migrated bi-directionally between dark and light zones. Notably, follicular B cells were frequent visitors to the germinal-centre compartment, suggesting that all B cells scan antigen trapped in germinal centres. Consistent with this observation, we found that high-affinity antigen-specific B cells can be recruited to an ongoing germinal-centre reaction. We conclude that the open structure of germinal centres enhances competition and ensures that rare high-affinity B cells can participate in antibody responses.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Jacob, J., Kelsoe, G., Rajewsky, K. & Weiss, U. Intraclonal generation of antibody mutants in germinal centres. Nature 354, 389–392 (1991)
Jacob, J. & Kelsoe, G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. II. A common clonal origin for periarteriolar lymphoid sheath-associated foci and germinal centers. J. Exp. Med. 176, 679–687 (1992)
Kroese, F. G., Wubbena, A. S., Seijen, H. G. & Nieuwenhuis, P. Germinal centers develop oligoclonally. Eur. J. Immunol. 17, 1069–1072 (1987)
Hanna, M. G. An autoradiographic study of the germinal center in spleen white pulp during early intervals of the immune response. Lab. Invest. 13, 95–104 (1964)
Nossal, G. J., Ada, G. L., Austin, C. M. & Pye, J. Antigens in immunity. 8. Localization of 125-I-labelled antigens in the secondary response. Immunology 9, 349–357 (1965)
MacLennan, I. C. Germinal centers. Annu. Rev. Immunol. 12, 117–139 (1994)
Rajewsky, K. Clonal selection and learning in the antibody system. Nature 381, 751–758 (1996)
Liu, Y. J. et al. Mechanism of antigen-driven selection in germinal centres. Nature 342, 929–931 (1989)
Miller, M. J., Wei, S. H., Cahalan, M. D. & Parker, I. Autonomous T cell trafficking examined in vivo with intravital two-photon microscopy. Proc. Natl Acad. Sci. USA 100, 2604–2609 (2003)
Mempel, T. R., Henrickson, S. E. & Von Andrian, U. H. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature 427, 154–159 (2004)
Lindquist, R. L. et al. Visualizing dendritic cell networks in vivo. Nature Immunol. 5, 1243–1250 (2004)
Shih, T. A., Roederer, M. & Nussenzweig, M. C. Role of antigen receptor affinity in T cell-independent antibody responses in vivo. Nature Immunol. 3, 399–406 (2002)
Schaefer, B. C., Schaefer, M. L., Kappler, J. W., Marrack, P. & Kedl, R. M. Observation of antigen-dependent CD8+ T-cell/dendritic cell interactions in vivo. Cell. Immunol. 214, 110–122 (2001)
Hadjantonakis, A. K., Macmaster, S. & Nagy, A. Embryonic stem cells and mice expressing different GFP variants for multiple non-invasive reporter usage within a single animal. BMC Biotechnol. 2, 11 (2002)
Kosco, M. H., Pflugfelder, E. & Gray, D. Follicular dendritic cell-dependent adhesion and proliferation of B cells in vitro. J. Immunol. 148, 2331–2339 (1992)
Szakal, A. K., Kosco, M. H. & Tew, J. G. A novel in vivo follicular dendritic cell-dependent iccosome-mediated mechanism for delivery of antigen to antigen-processing cells. J. Immunol. 140, 341–353 (1988)
Okada, T. et al. Antigen-engaged B cells undergo chemotaxis toward the T zone and form motile conjugates with helper T cells. PLoS Biol. 3, e150 (2005)
Miller, M. J., Wei, S. H., Parker, I. & Cahalan, M. D. Two-photon imaging of lymphocyte motility and antigen response in intact lymph node. Science 296, 1869–1873 (2002)
Han, S. B. et al. Rgs1 and Gnai2 regulate the entrance of B lymphocytes into lymph nodes and B cell motility within lymph node follicles. Immunity 22, 343–354 (2005)
Sumen, C., Mempel, T. R., Mazo, I. B. & von Andrian, U. H. Intravital microscopy: visualizing immunity in context. Immunity 21, 315–329 (2004)
Oprea, M. & Perelson, A. S. Somatic mutation leads to efficient affinity maturation when centrocytes recycle back to centroblasts. J. Immunol. 158, 5155–5162 (1997)
Fleire, S. J. et al. B cell ligand discrimination through a spreading and contraction response. Science 312, 738–741 (2006)
Qi, H., Egen, J. G., Huang, A. Y. & Germain, R. N. Extrafollicular activation of lymph node B cells by antigen-bearing dendritic cells. Science 312, 1672–1676 (2006)
Shakhar, G. et al. Stable T cell–dendritic cell interactions precede the development of both tolerance and immunity in vivo. Nature Immunol. 6, 707–714 (2005)
Fossum, S., Smith, M. E. & Ford, W. L. The recirculation of T and B lymphocytes in the athymic, nude rat. Scand. J. Immunol. 17, 551–557 (1983)
Bajenoff, M. et al. Stromal cell networks regulate lymphocyte entry, migration, and territoriality in lymph nodes. Immunity 25, 989–1001 (2006)
Jacob, J., Przylepa, J., Miller, C. & Kelsoe, G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. III. The kinetics of V region mutation and selection in germinal center B cells. J. Exp. Med. 178, 1293–1307 (1993)
Liu, Y. J., Zhang, J., Lane, P. J., Chan, E. Y. & MacLennan, I. C. Sites of specific B cell activation in primary and secondary responses to T cell-dependent and T cell-independent antigens. Eur. J. Immunol. 21, 2951–2962 (1991)
Kuppers, R., Zhao, M., Hansmann, M. L. & Rajewsky, K. Tracing B cell development in human germinal centres by molecular analysis of single cells picked from histological sections. EMBO J. 12, 4955–4967 (1993)
Deshmukh, U. S., Bagavant, H., Lewis, J., Gaskin, F. & Fu, S. M. Epitope spreading within lupus-associated ribonucleoprotein antigens. Clin. Immunol. 117, 112–120 (2005)
Acknowledgements
We thank E. Besmer, M. Zimmer and members of the Nussenzweig laboratory for comments on the manuscript. This work was supported by the Schering Foundation (T.A.S.), a Medical Scientist Training Program grant (R.L.L.), the Rothchild Foundation (G.S.), Fondation de Recherche Medicale (D.S.), and the NIH (M.C.N. and M.L.D.). M.C.N. is an investigator of the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
Competing interests: Marie Kosco-Vilbois works at Novlmmune, Geneva, as already stated.
Supplementary information
Supplementary Information
This file contains Supplementary Methods, Supplementary Figures S1-S8 and additional references. (PDF 7889 kb)
Supplementary Movie 1
This file contains Supplementary Movie 1 showing a time-lapse movie of a GC without FDC-M1. (MOV 2867 kb)
Supplementary Movie 2
This file contains Supplementary Movie 2 showing a time-lapse movie of GC B cells and Plasmablasts. (MOV 9775 kb)
Supplementary Movie 3
This file contains Supplementary Movie 3 showing a time-lapse movie of a B cell follicle without NP-OVA boost. (MOV 6240 kb)
Supplementary Movie 4
This file contains Supplementary Movie 4 showing a time-lapse movie of a GC. (MOV 6850 kb)
Supplementary Movie 5
This file contains Supplementary Movie 5 showing a two stacked time-lapse movie of a GC. (MOV 10942 kb)
Supplementary Movie 6
This file contains Supplementary Movie 6 showing a time-lapse movie of a WT B cell contacting FDC-M1+ cells. (MOV 3758 kb)
Corrigendum
In vivo imaging of germinal centres reveals a dynamic open structure Tanja A. Schwickert, Randall L. Lindquist, Guy Shakhar, Geulah Livshits, Dimitris Skokos, Marie H. Kosco-Vilbois, Michael L. Dustin and Michel C. Nussenzweig Nature doi:10.1038/nature 05573 (published online 31 January 2007) In Supplementary Movie 4 of this Letter, the LZ and DZ labels were swapped. The corrected version was uploaded on 5 February 2007. (PDF 11 kb)
Rights and permissions
About this article
Cite this article
Schwickert, T., Lindquist, R., Shakhar, G. et al. In vivo imaging of germinal centres reveals a dynamic open structure. Nature 446, 83–87 (2007). https://doi.org/10.1038/nature05573
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature05573
This article is cited by
-
Ultrasonic barrier-through imaging by Fabry-Perot resonance-tailoring panel
Nature Communications (2023)
-
Antigen presentation by B cells enables epitope spreading across an MHC barrier
Nature Communications (2023)
-
CXCL13 plasma levels function as a biomarker for disease activity in patients with chronic lymphocytic leukemia
Leukemia (2021)
-
Enhanced germinal center reaction by targeting vaccine antigen to major histocompatibility complex class II molecules
npj Vaccines (2019)
-
Immuno-engineered organoids for regulating the kinetics of B-cell development and antibody production
Nature Protocols (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.