Abstract
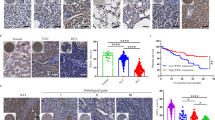
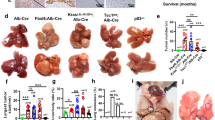
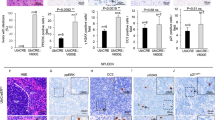
Although cancer arises from a combination of mutations in oncogenes and tumour suppressor genes, the extent to which tumour suppressor gene loss is required for maintaining established tumours is poorly understood. p53 is an important tumour suppressor that acts to restrict proliferation in response to DNA damage or deregulation of mitogenic oncogenes, by leading to the induction of various cell cycle checkpoints, apoptosis or cellular senescence1,2. Consequently, p53 mutations increase cell proliferation and survival, and in some settings promote genomic instability and resistance to certain chemotherapies3. To determine the consequences of reactivating the p53 pathway in tumours, we used RNA interference (RNAi) to conditionally regulate endogenous p53 expression in a mosaic mouse model of liver carcinoma4,5. We show that even brief reactivation of endogenous p53 in p53-deficient tumours can produce complete tumour regressions. The primary response to p53 was not apoptosis, but instead involved the induction of a cellular senescence program that was associated with differentiation and the upregulation of inflammatory cytokines. This program, although producing only cell cycle arrest in vitro, also triggered an innate immune response that targeted the tumour cells in vivo, thereby contributing to tumour clearance. Our study indicates that p53 loss can be required for the maintenance of aggressive carcinomas, and illustrates how the cellular senescence program can act together with the innate immune system to potently limit tumour growth.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Harris, S. L. & Levine, A. J. The p53 pathway: positive and negative feedback loops. Oncogene 24, 2899–2908 (2005)
Sherr, C. J. Principles of tumor suppression. Cell 116, 235–246 (2004)
Lowe, S. W., Cepero, E. & Evan, G. Intrinsic tumour suppression. Nature 432, 307–315 (2004)
Zender, L. et al. Generation and analysis of genetically defined liver carcinomas derived from bipotential liver progenitors. Cold Spring Harb. Symp. Quant. Biol. 70, 251–261 (2005)
Zender, L. et al. Identification and validation of oncogenes in liver cancer using an integrative oncogenomic approach. Cell 125, 1253–1267 (2006)
Staib, F., Hussain, S. P., Hofseth, L. J., Wang, X. W. & Harris, C. C. TP53 and liver carcinogenesis. Hum. Mutat. 21, 201–216 (2003)
Dickins, R. A. et al. Probing tumor phenotypes using stable and regulated synthetic microRNA precursors. Nature Genet. 37, 1289–1295 (2005)
Silva, J. M. et al. Second-generation shRNA libraries covering the mouse and human genomes. Nature Genet. 37, 1281–1288 (2005)
Chin, L. et al. Essential role for oncogenic Ras in tumour maintenance. Nature 400, 468–472 (1999)
Jain, M. et al. Sustained loss of a neoplastic phenotype by brief inactivation of MYC. Science 297, 102–104 (2002)
Braig, M. et al. Oncogene-induced senescence as an initial barrier in lymphoma development. Nature 436, 660–665 (2005)
Chen, Z. et al. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature 436, 725–730 (2005)
Collado, M. et al. Tumour biology: senescence in premalignant tumours. Nature 436, 642 (2005)
Michaloglou, C. et al. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature 436, 720–724 (2005)
Serrano, M., Lin, A. W., McCurrach, M. E., Beach, D. & Lowe, S. W. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88, 593–602 (1997)
Minamino, T. et al. Ras induces vascular smooth muscle cell senescence and inflammation in human atherosclerosis. Circulation 108, 2264–2269 (2003)
Shelton, D. N., Chang, E., Whittier, P. S., Choi, D. & Funk, W. D. Microarray analysis of replicative senescence. Curr. Biol. 9, 939–945 (1999)
Gorgoulis, V. G. et al. p53-dependent ICAM-1 overexpression in senescent human cells identified in atherosclerotic lesions. Lab. Invest. 85, 502–511 (2005)
Maeda, S., Kamata, H., Luo, J. L., Leffert, H. & Karin, M. IKKβ couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell 121, 977–990 (2005)
Mundt, B. et al. Involvement of TRAIL and its receptors in viral hepatitis. FASEB J. 17, 94–96 (2003)
Hong, F. et al. β-glucan functions as an adjuvant for monoclonal antibody immunotherapy by recruiting tumoricidal granulocytes as killer cells. Cancer Res. 63, 9023–9031 (2003)
Shultz, L. D. et al. Multiple defects in innate and adaptive immunologic function in NOD/LtSz-scid mice. J. Immunol. 154, 180–191 (1995)
Ghiringhelli, F., Menard, C., Martin, F. & Zitvogel, L. The role of regulatory T cells in the control of natural killer cells: relevance during tumor progression. Immunol. Rev. 214, 229–238 (2006)
Ventura, A. et al. Restoration of p53 function leads to tumour regression in vivo. Nature advance online publication, doi:10.1038/nature05541 (24 January 2007)
Christophorou, M. A., Ringshausen, I., Finch, A. J., Swigart, L. B. & Evan, G. I. The pathological response to DNA damage does not contribute to p53-mediated tumour suppression. Nature 443, 214–217 (2006)
Bykov, V. J. et al. Restoration of the tumor suppressor function to mutant p53 by a low-molecular-weight compound. Nature Med. 8, 282–288 (2002)
Vassilev, L. T. et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science 303, 844–848 (2004)
Gray-Schopfer, V. C. et al. Cellular senescence in naevi and immortalisation in melanoma: a role for p16?. Br. J. Cancer 95, 496–505 (2006)
Roninson, I. B. Tumor cell senescence in cancer treatment. Cancer Res. 63, 2705–2715 (2003)
Krtolica, A., Parrinello, S., Lockett, S., Desprez, P. Y. & Campisi, J. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging. Proc. Natl Acad. Sci. USA 98, 12072–12077 (2001)
Acknowledgements
We thank L. Bianco and M. Jiao for technical assistance. We also thank G. Evan, T. Jacks, A. Ventura, M. Narita, A. Chicas, M. Yon, G. Hannon and other members of the Lowe and Hannon laboratories for advice and discussions. We thank M. McCurrach for editorial assistance. W.X. is in the MCB graduate program at Stony Brook University. This work was generously supported by the Emmy Noether Programme of the German Research Foundation, Alan and Edith Seligson, the Don Monti Foundation, and grants from the National Institutes of Health (C.C.C, S.W.L.). This work is dedicated to our friend and colleague Dr. Enrique (Henry) Cepero.
Author Contributions W.X.: study design and conduction of experiments; L.Z.: study design and conduction of experiments; C.M.: design and conduction of flow cytometry experiments; R.A.D.: vector development; E.H.: histopathological analyses; V.K.: microarray analysis; C.C.C: histopathological analyses; S.W.L.: study design, principal investigator.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Reprints and permissions information is available at www.nature.com/reprints. The authors declare no competing financial interests.
Supplementary information
Supplementary information
This file contains Supplementary Methods , Supplementary figures S1-S9 with legends and additional references. (PDF 5545 kb)
Rights and permissions
About this article
Cite this article
Xue, W., Zender, L., Miething, C. et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature 445, 656–660 (2007). https://doi.org/10.1038/nature05529
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature05529
This article is cited by
-
Translating p53-based therapies for cancer into the clinic
Nature Reviews Cancer (2024)
-
Targeting aging and age-related diseases with vaccines
Nature Aging (2024)
-
Mutant p53 gains oncogenic functions through a chromosomal instability-induced cytosolic DNA response
Nature Communications (2024)
-
Pharmacological reactivation of p53 in the era of precision anticancer medicine
Nature Reviews Clinical Oncology (2024)
-
METTL3 promotes cellular senescence of colorectal cancer via modulation of CDKN2B transcription and mRNA stability
Oncogene (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.