Abstract
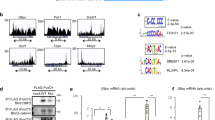
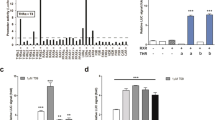
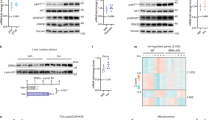
The liver has a central role in glucose homeostasis, as it has the distinctive ability to produce and consume glucose1. On feeding, glucose influx triggers gene expression changes in hepatocytes to suppress endogenous glucose production and convert excess glucose into glycogen or fatty acids to be stored in adipose tissue2. This process is controlled by insulin, although debate exists as to whether insulin acts directly or indirectly on the liver3. In addition to stimulating pancreatic insulin release, glucose also regulates the activity of ChREBP, a transcription factor that modulates lipogenesis4. Here we describe another mechanism whereby glucose determines its own fate: we show that glucose binds and stimulates the transcriptional activity of the liver X receptor (LXR), a nuclear receptor that coordinates hepatic lipid metabolism. d-Glucose and d-glucose-6-phosphate are direct agonists of both LXR-α and LXR-β. Glucose activates LXR at physiological concentrations expected in the liver and induces expression of LXR target genes with efficacy similar to that of oxysterols, the known LXR ligands. Cholesterol homeostasis genes that require LXR for expression are upregulated in liver and intestine of fasted mice re-fed with a glucose diet, indicating that glucose is an endogenous LXR ligand. Our results identify LXR as a transcriptional switch that integrates hepatic glucose metabolism and fatty acid synthesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Zierler, K. Whole body glucose metabolism. Am. J. Physiol. 276, E409–E426 (1999)
Moore, M. C., Cherrington, A. D. & Wasserman, D. H. Regulation of hepatic and peripheral glucose disposal. Best Pract. Res. Clin. Endocrinol. Metab. 17, 343–364 (2003)
Cherrington, A. D. The role of hepatic insulin receptors in the regulation of glucose production. J. Clin. Invest. 115, 1136–1139 (2005)
Yamashita, H. et al. A glucose-responsive transcription factor that regulates carbohydrate metabolism in the liver. Proc. Natl Acad. Sci. USA 98, 9116–9121 (2001)
Shulman, A. I. & Mangelsdorf, D. J. Retinoid x receptor heterodimers in the metabolic syndrome. N. Engl. J. Med. 353, 604–615 (2005)
Schultz, J. R. et al. Role of LXRs in control of lipogenesis. Genes Dev. 14, 2831–2838 (2000)
Peet, D. J. et al. Cholesterol and bile acid metabolism are impaired in mice lacking the nuclear oxysterol receptor LXRα. Cell 93, 693–704 (1998)
Janowski, B. A., Willy, P. J., Devi, T. R., Falck, J. R. & Mangelsdorf, D. J. An oxysterol signalling pathway mediated by the nuclear receptor LXRα. Nature 383, 728–731 (1996)
Joseph, S. B. et al. Synthetic LXR ligand inhibits the development of atherosclerosis in mice. Proc. Natl Acad. Sci. USA 99, 7604–7609 (2002)
Laffitte, B. A. et al. Activation of liver X receptor improves glucose tolerance through coordinate regulation of glucose metabolism in liver and adipose tissue. Proc. Natl Acad. Sci. USA 100, 5419–5424 (2003)
Cao, G. et al. Antidiabetic action of a liver x receptor agonist mediated by inhibition of hepatic gluconeogenesis. J. Biol. Chem. 278, 1131–1136 (2003)
Stehno-Bittel, L., Perez-Terzic, C. & Clapham, D. E. Diffusion across the nuclear envelope inhibited by depletion of the nuclear Ca2+ store. Science 270, 1835–1838 (1995)
Fehr, M., Lalonde, S., Ehrhardt, D. W. & Frommer, W. B. Live imaging of glucose homeostasis in nuclei of COS-7 cells. J. Fluoresc. 14, 603–609 (2004)
Shimomura, I. et al. Insulin selectively increases SREBP-1c mRNA in the livers of rats with streptozotocin-induced diabetes. Proc. Natl Acad. Sci. USA 96, 13656–13661 (1999)
Eberle, D., Hegarty, B., Bossard, P., Ferre, P. & Foufelle, F. SREBP transcription factors: master regulators of lipid homeostasis. Biochimie 86, 839–848 (2004)
Liang, G. et al. Diminished hepatic response to fasting/refeeding and liver X receptor agonists in mice with selective deficiency of sterol regulatory element-binding protein-1c. J. Biol. Chem. 277, 9520–9528 (2002)
Repa, J. J. et al. Regulation of mouse sterol regulatory element-binding protein-1c gene (SREBP-1c) by oxysterol receptors, LXRα and LXRα. Genes Dev. 14, 2819–2830 (2000)
Chen, G., Liang, G., Ou, J., Goldstein, J. L. & Brown, M. S. Central role for liver X receptor in insulin-mediated activation of Srebp-1c transcription and stimulation of fatty acid synthesis in liver. Proc. Natl Acad. Sci. USA 101, 11245–11250 (2004)
Iizuka, K., Bruick, R. K., Liang, G., Horton, J. D. & Uyeda, K. Deficiency of carbohydrate response element-binding protein (ChREBP) reduces lipogenesis as well as glycolysis. Proc. Natl Acad. Sci. USA 101, 7281–7286 (2004)
Parks, E. J. Changes in fat synthesis influenced by dietary macronutrient content. Proc. Nutr. Soc. 61, 281–286 (2002)
Lin, J. et al. Hyperlipidemic effects of dietary saturated fats mediated through PGC-1β coactivation of SREBP. Cell 120, 261–273 (2005)
Joseph, S. B. et al. LXR-dependent gene expression is important for macrophage survival and the innate immune response. Cell 119, 299–309 (2004)
Janowski, B. A. et al. Structural requirements of ligands for the oxysterol liver X receptors LXRα and LXRβ. Proc. Natl Acad. Sci. USA 96, 266–271 (1999)
DeBlasi, A., O’Reilly, K. & Motulsky, H. J. Calculating receptor number from binding experiments using same compound as radioligand and competitor. Trends Pharmacol. Sci. 10, 227–229 (1989)
Acknowledgements
We thank S. Bohan, R. Romeo, B. Geierstanger, M. Chalmers, P. Griffin, N. Gekakis, M. Crestani, H. Shimano, T. Matsuzaka, P. Tontonoz, B. Laffitte, S. Joseph, A. Brock, E. Peters and K. Nettles for technical help and/or useful comments. C.G. was a visiting scientist supported by a fellowship from the Department of Pharmacological Sciences, University of Milano, Italy.
Author Contributions L.V., C.G., E.H. and A.K. performed experiments; P.A.M. and V.M. designed and performed experiments and analysed data; and N.M. and E.S. designed and performed experiments, analysed data, and wrote the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Reprints and permissions information is available at www.nature.com/reprints. The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-10 with legends, Supplementary Methods and Supplementary Table 1. (PDF 962 kb)
Rights and permissions
About this article
Cite this article
Mitro, N., Mak, P., Vargas, L. et al. The nuclear receptor LXR is a glucose sensor. Nature 445, 219–223 (2007). https://doi.org/10.1038/nature05449
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature05449
This article is cited by
-
Engelheptanoxides behave as liver X receptor α agonists
Medicinal Chemistry Research (2023)
-
An extended gate field-effect transistor (EG-FET) type non-enzymatic glucose sensor with inkjet-printed copper oxide nanoparticles
SN Applied Sciences (2022)
-
Advances in fatty acids nutrition in dairy cows: from gut to cells and effects on performance
Journal of Animal Science and Biotechnology (2020)
-
6-Ketocholestanol suppresses lipid accumulation by decreasing FASN gene expression through SREBP-dependent regulation in HepG2 cells
Cytotechnology (2020)
-
Stress as an immunomodulator: liver X receptors maybe the answer
Inflammopharmacology (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.