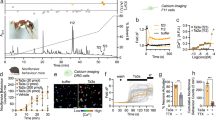
Abstract
Bites and stings from venomous creatures can produce pain and inflammation as part of their defensive strategy to ward off predators or competitors1,2. Molecules accounting for lethal effects of venoms have been extensively characterized, but less is known about the mechanisms by which they produce pain. Venoms from spiders, snakes, cone snails or scorpions contain a pharmacopoeia of peptide toxins that block receptor or channel activation as a means of producing shock, paralysis or death3,4,5. We examined whether these venoms also contain toxins that activate (rather than inhibit) excitatory channels on somatosensory neurons to produce a noxious sensation in mammals. Here we show that venom from a tarantula that is native to the West Indies contains three inhibitor cysteine knot (ICK) peptides that target the capsaicin receptor (TRPV1), an excitatory channel expressed by sensory neurons of the pain pathway6. In contrast with the predominant role of ICK toxins as channel inhibitors5,7, these previously unknown ‘vanillotoxins’ function as TRPV1 agonists, providing new tools for understanding mechanisms of TRP channel gating. Some vanillotoxins also inhibit voltage-gated potassium channels, supporting potential similarities between TRP and voltage-gated channel structures. TRP channels can now be included among the targets of peptide toxins, showing that animals, like plants (for example, chilli peppers), avert predators by activating TRP channels on sensory nerve fibres to elicit pain and inflammation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Schmidt, J. O. Biochemistry of insect venoms. Annu. Rev. Entomol. 27, 339–368 (1982)
Chahl, L. A. & Kirk, E. J. Toxins which produce pain. Pain 1, 3–49 (1975)
Escoubas, P. & Rash, L. Tarantulas: eight-legged pharmacists and combinatorial chemists. Toxicon 43, 555–574 (2004)
Miller, C. The charybdotoxin family of K+ channel-blocking peptides. Neuron 15, 5–10 (1995)
Terlau, H. & Olivera, B. M. Conus venoms: a rich source of novel ion channel-targeted peptides. Physiol. Rev. 84, 41–68 (2004)
Caterina, M. J. et al. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389, 816–824 (1997)
Swartz, K. J. & MacKinnon, R. An inhibitor of the Kv2.1 potassium channel isolated from the venom of a Chilean tarantula. Neuron 15, 941–949 (1995)
Jordt, S. E., McKemy, D. D. & Julius, D. Lessons from peppers and peppermint: the molecular logic of thermosensation. Curr. Opin. Neurobiol. 13, 487–492 (2003)
Voets, T., Talavera, K., Owsianik, G. & Nilius, B. Sensing with TRP channels. Nature Chem. Biol. 1, 85–92 (2005)
Zhu, S., Darbon, H., Dyason, K., Verdonck, F. & Tytgat, J. Evolutionary origin of inhibitor cystine knot peptides. FASEB J. 17, 1765–1767 (2003)
Swartz, K. J. & MacKinnon, R. Mapping the receptor site for hanatoxin, a gating modifier of voltage-dependent K+ channels. Neuron 18, 675–682 (1997)
Oswald, R. E., Suchyna, T. M., McFeeters, R., Gottlieb, P. & Sachs, F. Solution structure of peptide toxins that block mechanosensitive ion channels. J. Biol. Chem. 277, 34443–34450 (2002)
Takahashi, H. et al. Solution structure of hanatoxin1, a gating modifier of voltage-dependent K+ channels: common surface features of gating modifier toxins. J. Mol. Biol. 297, 771–780 (2000)
Wang, J. M. et al. Molecular surface of tarantula toxins interacting with voltage sensors in Kv channels. J. Gen. Physiol. 123, 455–467 (2004)
Escoubas, P., Diochot, S., Celerier, M. L., Nakajima, T. & Lazdunski, M. Novel tarantula toxins for subtypes of voltage-dependent potassium channels in the Kv2 and Kv4 subfamilies. Mol. Pharmacol. 62, 48–57 (2002)
Zhang, P. F., Chen, P., Hu, W. J. & Liang, S. P. Huwentoxin-V, a novel insecticidal peptide toxin from the spider Selenocosmia huwena, and a natural mutant of the toxin: indicates the key amino acid residues related to the biological activity. Toxicon 42, 15–20 (2003)
Voets, T. et al. The principle of temperature-dependent gating in cold- and heat-sensitive TRP channels. Nature 430, 748–754 (2004)
Jung, J. et al. Capsaicin binds to the intracellular domain of the capsaicin-activated ion channel. J. Neurosci. 19, 529–538 (1999)
Jordt, S. E. & Julius, D. Molecular basis for species-specific sensitivity to ‘hot’ chili peppers. Cell 108, 421–430 (2002)
Gavva, N. R. et al. Molecular determinants of vanilloid sensitivity in TRPV1. J. Biol. Chem. 279, 20283–20295 (2004)
Salinas, M. et al. The receptor site of the spider toxin PcTx1 on the proton-gated cation channel ASIC1a. J. Physiol. (Lond.) 570, 339–354 (2006)
Lee, S. Y. & MacKinnon, R. A membrane-access mechanism of ion channel inhibition by voltage sensor toxins from spider venom. Nature 430, 232–235 (2004)
Suchyna, T. M. et al. Bilayer-dependent inhibition of mechanosensitive channels by neuroactive peptide enantiomers. Nature 430, 235–240 (2004)
Tominaga, M. et al. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron 21, 531–543 (1998)
Ramu, Y., Xu, Y. & Lu, Z. Enzymatic activation of voltage-gated potassium channels. Nature 442, 696–699 (2006)
Ramsey, I. S., Delling, M. & Clapham, D. E. An introduction to TRP channels. Annu. Rev. Physiol. 68, 619–647 (2006)
Phillips, L. R. et al. Voltage-sensor activation with a tarantula toxin as cargo. Nature 436, 857–860 (2005)
Jordt, S. E. et al. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 427, 260–265 (2004)
Hamill, O. P., Marty, A., Neher, E., Sakmann, B. & Sigworth, F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch. 391, 85–100 (1981)
Martinez-Caro, L. & Laird, J. M. Allodynia and hyperalgesia evoked by sciatic mononeuropathy in NKI receptor knockout mice. Neuroreport 11, 1213–1217 (2000)
Acknowledgements
We thank D. Minor and members of his laboratory for Kv4.2 and Kchip complementary RNAs and advice with chromatographic methods; D. Clapham and R. Aldrich for providing TRPV3 and Kv2.1 plasmids, respectively; K. Shokat and J. Blethrow for initial help with mass spectrometry; members of the Julius laboratory for discussions; and R. Nicoll, R. Edwards and H. Chuang for critical reading of the manuscript. This work was supported by NIH grants (to D.J., A.I.B. and E.A.L.) and by postdoctoral fellowships from the Swiss National Science Foundation, Novartis Stiftung, and the International Human Frontier Science Program Organization (to J.S.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Reprints and permissions information is available at www.nature.com/reprints. The authors declare no competing financial interests.
Supplementary information
Supplementary Notes
This file contains Supplementary Figures 1–7. Supplementary Figure 1 shows a representative chromatogram of vanillotoxin purification. Displayed are HPLC chromatograms and assessment of activity by ratiometric calcium imaging. Toxin purification is also described in detail. Supplementary Figures 2 and 3 describe the synthesis of VaTx1. Calcium imaging data display specific activation of the capsaicin receptor, TRPV1, by native and synthetic vanillotoxins. Supplementary Figures 4–6 extend the electrophysiological analysis of vanillotoxin effects on TRPV1 and on the voltage-gated potassium channel, Kv2.1 (see Figure 2 and 3 of the main manuscript). Supplementary Figure 7 displays the effects of crude Psalmopoeus cambridgei venom on trigeminal neurons cultured from wild-type or TRPV1-deficient mice, as assessed by ratiometric calcium imaging. (PDF 3060 kb)
Rights and permissions
About this article
Cite this article
Siemens, J., Zhou, S., Piskorowski, R. et al. Spider toxins activate the capsaicin receptor to produce inflammatory pain. Nature 444, 208–212 (2006). https://doi.org/10.1038/nature05285
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature05285
This article is cited by
-
TRPV1-Targeted Drugs in Development for Human Pain Conditions
Drugs (2021)
-
Peripheral receptors and neuromediators involved in the antihyperalgesic effects of acupuncture: a state-of-the-art review
Pflügers Archiv - European Journal of Physiology (2021)
-
Silencing of spinal Trpv1 attenuates neuropathic pain in rats by inhibiting CAMKII expression and ERK2 phosphorylation
Scientific Reports (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.