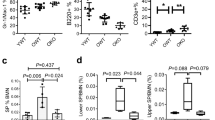
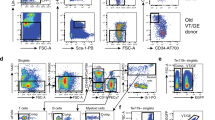
Abstract
Stem-cell ageing is thought to contribute to altered tissue maintenance and repair. Older humans experience increased bone marrow failure and poorer haematologic tolerance of cytotoxic injury. Haematopoietic stem cells (HSCs) in older mice have decreased per-cell repopulating activity, self-renewal and homing abilities, myeloid skewing of differentiation, and increased apoptosis with stress. Here we report that the cyclin-dependent kinase inhibitor p16INK4a, the level of which was previously noted to increase in other cell types with age, accumulates and modulates specific age-associated HSC functions. Notably, in the absence of p16INK4a, HSC repopulating defects and apoptosis were mitigated, improving the stress tolerance of cells and the survival of animals in successive transplants, a stem-cell-autonomous tissue regeneration model. Inhibition of p16INK4a may ameliorate the physiological impact of ageing on stem cells and thereby improve injury repair in aged tissue.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Cheng, T. et al. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science 287, 1804–1808 (2000)
Walkley, C. R., Fero, M. L., Chien, W. M., Purton, L. E. & McArthur, G. A. Negative cell-cycle regulators cooperatively control self-renewal and differentiation of haematopoietic stem cells. Nature Cell Biol. 7, 172–178 (2005)
Yuan, Y., Shen, H., Franklin, D. S., Scadden, D. T. & Cheng, T. In vivo self-renewing divisions of haematopoietic stem cells are increased in the absence of the early G1-phase inhibitor, p18INK4C. Nature Cell Biol. 6, 436–442 (2004)
Sharpless, N. E. Ink4a/Arf links senescence and aging. Exp. Gerontol. 39, 1751–1759 (2004)
Rocco, J. W. & Sidransky, D. p16(MTS-1/CDKN2/INK4a) in cancer progression. Exp. Cell Res. 264, 42–55 (2001)
Krishnamurthy, J. et al. Ink4a/Arf expression is a biomarker of aging. J. Clin. Invest. 114, 1299–1307 (2004)
Zindy, F., Quelle, D. E., Roussel, M. F. & Sherr, C. J. Expression of the p16INK4a tumour suppressor versus other INK4 family members during mouse development and aging. Oncogene 15, 203–211 (1997)
Campisi, J. Cellular senescence as a tumour-suppressor mechanism. Trends Cell Biol. 11, S27–S31 (2001)
Chen, J., Astle, C. M. & Harrison, D. E. Development and aging of primitive hematopoietic stem cells in BALB/cBy mice. Exp. Hematol. 27, 928–935 (1999)
Ogden, D. A. & Mickliem, H. S. The fate of serially transplanted bone marrow cell populations from young and old donors. Transplantation 22, 287–293 (1976)
Morrison, S. J., Wandycz, A. M., Akashi, K., Globerson, A. & Weissman, I. L. The aging of hematopoietic stem cells. Nature Med. 2, 1011–1016 (1996)
Liang, Y., Van Zant, G. & Szilvassy, S. J. Effects of aging on the homing and engraftment of murine hematopoietic stem and progenitor cells. Blood 106, 1479–1487 (2005)
Osawa, M., Hanada, K., Hamada, H. & Nakauchi, H. Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science 273, 242–245 (1996)
Adolfsson, J. et al. Identification of Flt3+ lympho-myeloid stem cells lacking erythro-megakaryocytic potential. A revised road map for adult blood lineage commitment. Cell 121, 295–306 (2005)
Sharpless, N. E. et al. Loss of p16Ink4a with retention of p19Arf predisposes mice to tumorigenesis. Nature 413, 86–91 (2001)
Yilmaz, O. H., Kiel, M. J. & Morrison, S. J. SLAM family markers are conserved among hematopoietic stem cells from old and reconstituted mice and markedly increase their purity. Blood 107, 924–930 (2006)
Wagers, A. J., Sherwood, R. I., Christensen, J. L. & Weissman, I. L. Little evidence for developmental plasticity of adult hematopoietic stem cells. Science 297, 2256–2259 (2002)
Morrison, S. J., Wright, D. E. & Weissman, I. L. Cyclophosphamide/granulocyte colony-stimulating factor induces hematopoietic stem cells to proliferate prior to mobilization. Proc. Natl Acad. Sci. USA 94, 1908–1913 (1997)
Molofsky, A.V. et al. Increasing p16INK4a expression decreases forebrain progenitors and neurogenesis during ageing. Nature advance online publication, doi:10.1038/nature05091 (6 September 2006).
Siminovitch, L., Till, J. E. & McCulloch, E. A. Decline in colony-forming ability of marrow cells subjected to serial transplantation into irradiated mice. J. Cell. Physiol. 64, 23–31 (1964)
Harrison, D. E. Normal function of transplanted mouse erythrocyte precursors for 21 months beyond donor life spans. Nature New Biol. 237, 220–222 (1972)
Chen, J., Astle, C. M. & Harrison, D. E. Genetic regulation of primitive hematopoietic stem cell senescence. Exp. Hematol. 28, 442–450 (2000)
Domen, J., Cheshier, S. H. & Weissman, I. L. The role of apoptosis in the regulation of hematopoietic stem cells: overexpression of BCL-2 increases both their number and repopulation potential. J. Exp. Med. 191, 253–264 (2000)
Chkhotua, A. B. et al. Increased expression of p16(INK4a) and p27(Kip1) cyclin-dependent kinase inhibitor genes in aging human kidney and chronic allograft nephropathy. Am. J. Kidney Dis. 41, 1303–1313 (2003)
Chimenti, C. et al. Senescence and death of primitive cells and myocytes lead to premature cardiac aging and heart failure. Circ. Res. 93, 604–613 (2003)
Park, I. K. et al. Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. Nature 423, 302–305 (2003)
Stepanova, L. & Sorrentino, B. P. A limited role for p16Ink4a and p19Arf in the loss of hematopoietic stem cells during proliferative stress. Blood 106, 827–832 (2005)
Kunisato, A. et al. HES-1 preserves purified hematopoietic stem cells ex vivo and accumulates side population cells in vivo. Blood 101, 1777–1783 (2003)
Krishnamurthy, J. et al. p16INK4a induces an age-dependent decline in islet regenerative potential. Nature advance online publication, doi:10.1038/nature05092 (6 September 2006).
Phelps, W. C., Munger, K., Yee, C. L., Barnes, J. A. & Howley, P. M. Structure–function analysis of the human papilloma virus type 16 E7 oncoprotein. J. Virol. 66, 2418–2427 (1992)
Conboy, I. M. et al. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 433, 760–764 (2005)
Stier, S., Cheng, T., Dombkowski, D., Carlesso, N. & Scadden, D. T. Notch1 activation increases hematopoietic stem cell self-renewal in vivo and favors lymphoid over myeloid lineage outcome. Blood 99, 2369–2378 (2002)
Adams, G. B. et al. Stem cell engraftment at the endosteal niche is specified by the calcium-sensing receptor. Nature 439, 599–603 (2006)
Acknowledgements
We thank K. Munger for the HPV16 E7 clones. We also thank the National Institutes of Health (D.T.S., H.E.F., Y.S., T.C., R.A.D. and N.E.S.), Dr. Mildred Scheel Stiftung fuer Krebsforschung (V.J.), Deutsche Forschungsgemeinschaft (R.F.), The Ellison Medical Foundation and American Cancer Society (R.A.D.), The Paul Beeson Program in Aging Research (N.E.S.), The Sidney Kimmel Foundation (N.E.S.) and The Burroughs Wellcome Foundation (D.T.S.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Reprints and permissions information is available at www.nature.com/reprints. The authors declare no competing financial interests.
Supplementary information
Supplementary Figures
This file contains Supplementary Figures 1–8. (PPT 1056 kb)
Supplementary Figures
This file contains text to accompany the above Supplementary Figures. (DOC 29 kb)
Rights and permissions
About this article
Cite this article
Janzen, V., Forkert, R., Fleming, H. et al. Stem-cell ageing modified by the cyclin-dependent kinase inhibitor p16INK4a. Nature 443, 421–426 (2006). https://doi.org/10.1038/nature05159
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature05159
This article is cited by
-
Ppm1d truncating mutations promote the development of genotoxic stress-induced AML
Leukemia (2023)
-
Prion infection modulates hematopoietic stem/progenitor cell fate through cell-autonomous and non-autonomous mechanisms
Leukemia (2023)
-
CD133+ endothelial-like stem cells restore neovascularization and promote longevity in progeroid and naturally aged mice
Nature Aging (2023)
-
Differences in the post-stroke innate immune response between young and old
Seminars in Immunopathology (2023)
-
Cellular senescence and neurodegeneration
Human Genetics (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.