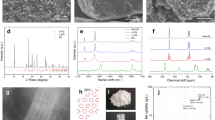
Abstract
The challenges associated with synthesizing expanded semiconductor frameworks with cage-like crystal structures continue to be of interest1,2. Filled low-density germanium and silicon framework structures have distinct properties that address important issues in thermoelectric phonon glass–electron crystals3, superconductivity4 and the possibility of Kondo insulators5. Interest in empty framework structures of silicon and germanium is motivated by their predicted wide optical bandgaps of the same magnitude as quantum dots and porous silicon, making them and their alloys promising materials for silicon-based optoelectronic devices6,7. Although almost-empty Na1-xSi136 has already been reported8,9, the synthesis of guest-free germanium clathrate has so far been unsuccessful. Here we report the high-yield synthesis and characteristics of germanium with the empty clathrate-II structure through the oxidation of  Zintl anions in ionic liquids under ambient conditions. The approach demonstrates the potential of ionic liquids as media for the reactions of polar intermetallic phases.
Zintl anions in ionic liquids under ambient conditions. The approach demonstrates the potential of ionic liquids as media for the reactions of polar intermetallic phases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Kasper, J. S., Hagenmuller, P., Pouchard, M. & Cros, C. Clathrate structure of silicon Na8Si46 and NaxSi136 (x < 11). Science 150, 1713–1714 (1965)
Trikalitis, P. N., Rangan, K. K., Bakas, T. & Kanatzidis, M. G. Varied pore organization in mesostructured semiconductors based on the [SnSe4]4- anion. Nature 410, 671–675 (2001)
Cohn, J. L., Nolas, G. S., Fessatidis, V., Metcalf, T. H. & Slack, G. A. Glasslike heat conduction in high-mobility crystalline semiconductors. Phys. Rev. Lett. 82, 779–782 (1999)
Connétable, D. et al. Superconductivty in doped sp3 semiconductors: The case of the clathrates. Phys. Rev. Lett. 91, 247001 (2003)
Paschen, S. et al. Towards strongly correlated semimetals: U2Ru2Sn and Eu8Ga16Ge30 . J. Phys. Chem. Solids 63, 1183–1188 (2002)
Moriguchi, K., Munetoh, S. & Shintani, A. First-principles study of Si34-xGex clathrates: Direct wide-gap semiconductors in Si-Ge alloys. Phys. Rev. B 62, 7138–7143 (2000)
Adams, G. B., O'Keeffe, M., Demkov, A. A., Sankey, O. F. & Huang, Y.-M. Wide-band-gap Si in open fourfold-coordinated clathrate structures. Phys. Rev. B 49, 8048–8053 (1994)
Gryko, J. et al. Low-density framework form of crystalline silicon with a wide optical band gap. Phys. Rev. B 62, 7707–7710 (2000)
Ammar, A. et al. On the clathrate form of elemental silicon, Si136: preparation and characterisation of NaxSi136 (x → 0). Solid State Sci. 6, 393–400 (2004)
Cundy, C. S. & Cox, P. A. The hydrothermal synthesis of zeolites: History and development from the earliest days to the present time. Chem. Rev. 103, 663–701 (2003)
Feng, P., Bu, X. & Zheng, N. The interface chemistry between chalcogenide clusters and open framework chalcogenides. Acc. Chem. Res. 38, 293–303 (2005)
Grüttner, A., Nesper, R. & Von Schnering, H. G. Novel metastable germanium modifications allo-Ge and 4H-Ge from Li17Ge12 . Angew. Chem. Int. Edn Engl. 21, 912–913 (1982)
Von Schnering, H. G., Schwarz, M. & Nesper, R. The lithium sodium silicide Li3NaSi6 and the formation of allo-silicon. J. Less-Common Metals 137, 297–310 (1988)
Wilson, M. & McMillan, P. F. Crystal-liquid phase relations in silicon at negative pressure. Phys. Rev. Lett. 90, 135703 (2003)
Munetoh, S., Moriguchi, K., Kamei, K., Shintani, A. & Motooka, T. Epitaxial growth of a low-density framework form of crystalline silicon: A molecular-dynamics study. Phys. Rev. Lett. 86, 4879–4882 (2001)
Dong, J. & Sankey, O. F. Theoretical study of two expanded phases of crystalline germanium: clathrate-I and clathrate-II. J. Phys. Condens. Matter 11, 6129–6145 (1999)
de A. A. Soler-Illia, G. J., Sanchez, C., Lebeau, B. & Patarin, J. Chemical strategies to design textured materials: from microporous and mesoporous oxides to nanonetworks and hierarchical structures. Chem. Rev. 102, 4093–4138 (2002)
Von Schnering, H. G. et al. The cluster anion Si94-. Angew. Chem. Int. Edn Engl. 37, 2359–2361 (1998)
Quéneau, V. & Sevov, S. C. Ge94-: A deltahedral Zintl ion made in the solid state. Angew. Chem. Int. Edn Engl. 36, 1754–1756 (1997)
Downie, C., Tang, Z. & Guloy, A. M. An unprecedented ∞1[Ge9]2- polymer: A link between molecular Zintl clusters and solid-state phases. Angew. Chem. Int. Edn Engl. 39, 338–340 (2000)
Downie, C., Mao, J.-G., Parmar, H. & Guloy, A. M. The role of sequestering agents in the formation and structure of germanium anion cluster polymers. Inorg. Chem. 43, 1992–1997 (2004)
McGrath, K. M. Phase behavior of dodecyltrimethylammonium bromide/water mixtures. Langmuir 11, 1835–1839 (1995)
Cooper, E. R. et al. Ionic liquids and eutectic mixtures as solvent and template in synthesis of zeolite analogues. Nature 430, 1012–1016 (2004)
Grovenstein, E. Jr & Stevenson, R. W. Carbanions. III. Cleavage of tetraalkylammonium halides by sodium in liquid ammonia. J. Am. Chem. Soc. 81, 4850–4857 (1959)
Cros, C., Pouchard, M. & Hagenmuller, P. Sur une nouvelle famille de clathrates minéraux isotypes des hydrates de gaz et de liquides. Interprétation des résultats obtenus. J. Solid State Chem. 2, 570–581 (1970)
Gryko, J. et al. Electron structure and temperature-dependent shifts in 133Cs NMR spectra of Cs8Ge136 clathrate. Phys. Rev. B 71, 115208 (2005)
Bobev, S. & Sevov, S. C. Synthesis and characterization of stable clathrates of silicon and germanium: Cs8Na16Si136 and Cs8Na16Ge136 . J. Am. Chem. Soc. 121, 3795–3796 (1999)
Von Stackelberg, M. & Müller, H. R. Feste Gashydrate. II. Struktur und Raumchemie. Z. Elektrochem. 58, 25–39 (1954)
Gies, H., Liebau, F. & Gerke, H. ‘Dodecasile’—eine neue Reihe polytyper Einschlussverbindungen von SiO2 . Angew. Chem. 94, 214–215 (1982)
Von Schnering, H. G. Zintl phases: Principles of structure and bonding. Bol. Soc. Chil. Quim. 33, 41–57 (1988)
Acknowledgements
We thank R. Kniep and U. Schwarz for comments, R. Cardoso-Gil for XRPD measurements, G. Auffermann and U. Schmidt for chemical analysis, S. Müller for differential scanning calorimetry measurements, R. Koban for resistivity and susceptibility measurements, and P. Simon and W. Carrillo-Cabrera for discussion. A.M.G. thanks the PRF-ACS and the R. A. Welch Foundation for financial support. Author Contributions All authors contributed equally to this work. A.M.G., Z.T. and M.B. developed the synthetic method and prepared the clathrate material. Yu.G. solved the structure from X-ray diffraction data. R.R. performed electron microscopy investigations. W.S. made measurements of electrical conductivity and magnetic susceptibility, Z.T. performed diffuse reflectance measurements. All authors discussed the results and contributed to the final manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
Reprints and permissions information is available at www.nature.com/reprints. The authors declare no competing financial interests.
Supplementary information
Supplementary Notes
This file contains schematic representation of the synthesis of empty clathrate, crystallographic information, results of the measurements of electrical resistivity, magnetic susceptibility, optical absorption spectra, and additional references for used data evaluation methods and software. (DOC 956 kb)
Rights and permissions
About this article
Cite this article
Guloy, A., Ramlau, R., Tang, Z. et al. A guest-free germanium clathrate. Nature 443, 320–323 (2006). https://doi.org/10.1038/nature05145
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature05145
This article is cited by
-
Neon encapsulation by a hydroquinone organic crystalline clathrate under ambient conditions
Communications Materials (2023)
-
Stability of mixed carbon–silicon clathrates
Applied Physics A (2022)
-
Computational strategies for design and discovery of nanostructured thermoelectrics
npj Computational Materials (2019)
-
Formation of inclusion type silicon phases induced by inert gases
Communications Chemistry (2018)
-
Properties of the exotic metastable ST12 germanium allotrope
Nature Communications (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.