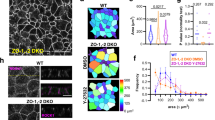
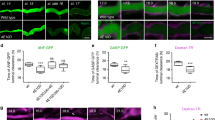
Abstract
Epithelial tissues maintain a robust architecture during development. This fundamental property relies on intercellular adhesion through the formation of adherens junctions containing E-cadherin molecules1,2. Localization of E-cadherin is stabilized through a pathway involving the recruitment of actin filaments by E-cadherin3,4,5,6. Here we identify an additional pathway that organizes actin filaments in the apical junctional region (AJR) where adherens junctions form in embryonic epithelia. This pathway is controlled by Bitesize (Btsz), a synaptotagmin-like protein7,8 that is recruited in the AJR independently of E-cadherin and is required for epithelial stability in Drosophila embryos. On loss of btsz, E-cadherin is recruited normally to the AJR, but is not stabilized properly and actin filaments fail to form a stable continuous network. In the absence of E-cadherin, actin filaments are stable for a longer time than they are in btsz mutants. We identify two polarized cues that localize Btsz: phosphatidylinositol (4,5)-bisphosphate, to which Btsz binds; and Par-3. We show that Btsz binds to the Ezrin–Radixin–Moesin protein Moesin, an F-actin-binding protein that is localized apically9 and is recruited in the AJR in a btsz-dependent manner. Expression of a dominant-negative form of Ezrin that does not bind F-actin phenocopies the loss of btsz. Thus, our data indicate that, through their interaction, Btsz and Moesin may mediate the proper organization of actin in a local domain, which in turn stabilizes E-cadherin. These results provide a mechanism for the spatial order of actin organization underlying junction stabilization in primary embryonic epithelia.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Gumbiner, B. M. Regulation of cadherin adhesive activity. J. Cell Biol. 148, 399–404 (2000)
Knust, E. & Bossinger, O. Composition and formation of intercellular junctions in epithelial cells. Science 298, 1955–1959 (2002)
Jamora, C. & Fuchs, E. Intercellular adhesion, signalling and the cytoskeleton. Nature Cell Biol. 4, E101–E108 (2002)
Bershadsky, A. Magic touch: how does cell–cell adhesion trigger actin assembly? Trends Cell Biol. 14, 589–593 (2004)
Drees, F., Pokutta, S., Yamada, S., Nelson, W. J. & Weis, W. I. α-Catenin is a molecular switch that binds E-cadherin–β-catenin and regulates actin-filament assembly. Cell 123, 903–915 (2005)
Yamada, S., Pokutta, S., Drees, F., Weis, W. I. & Nelson, W. J. Deconstructing the cadherin–catenin–actin complex. Cell 123, 889–901 (2005)
Fukuda, M., Saegusa, C. & Mikoshiba, K. Novel splicing isoforms of synaptotagmin-like proteins 2 and 3: identification of the Slp homology domain. Biochem. Biophys. Res. Commun. 283, 513–519 (2001)
Serano, J. & Rubin, G. M. The Drosophila synaptotagmin-like protein bitesize is required for growth and has mRNA localization sequences within its open reading frame. Proc. Natl Acad. Sci. USA 100, 13368–13373 (2003)
Bretscher, A., Edwards, K. & Fehon, R. G. ERM proteins and merlin: integrators at the cell cortex. Nature Rev. Mol. Cell Biol. 3, 586–599 (2002)
Kobielak, A., Pasolli, H. A. & Fuchs, E. Mammalian formin-1 participates in adherens junctions and polymerization of linear actin cables. Nature Cell Biol. 6, 21–30 (2004)
Nelson, W. J. Adaptation of core mechanisms to generate cell polarity. Nature 422, 766–774 (2003)
Harris, T. J. & Peifer, M. Adherens junction-dependent and-independent steps in the establishment of epithelial cell polarity in Drosophila. J. Cell Biol. 167, 135–147 (2004)
Tepass, U. et al. shotgun encodes Drosophila E-cadherin and is preferentially required during cell rearrangement in the neurectoderm and other morphogenetically active epithelia. Genes Dev. 10, 672–685 (1996)
Uemura, T. et al. Zygotic Drosophila E-cadherin expression is required for processes of dynamic epithelial cell rearrangement in the Drosophila embryo. Genes Dev. 10, 659–671 (1996)
Cox, R. T., Kirkpatrick, C. & Peifer, M. Armadillo is required for adherens junction assembly, cell polarity, and morphogenesis during Drosophila embryogenesis. J. Cell Biol. 134, 133–148 (1996)
Peifer, M., Orsulic, S., Sweeton, D. & Wieschaus, E. A role for the Drosophila segment polarity gene armadillo in cell adhesion and cytoskeletal integrity during oogenesis. Development 118, 1191–1207 (1993)
Petronczki, M. & Knoblich, J. A. DmPAR-6 directs epithelial polarity and asymmetric cell division of neuroblasts in Drosophila. Nature Cell Biol. 3, 43–49 (2001)
Muller, H. A. & Wieschaus, E. armadillo, bazooka, and stardust are critical for early stages in formation of the zonula adherens and maintenance of the polarized blastoderm epithelium in Drosophila. J. Cell Biol. 134, 149–163 (1996)
Wodarz, A., Ramrath, A., Grimm, A. & Knust, E. Drosophila atypical protein kinase C associates with Bazooka and controls polarity of epithelia and neuroblasts. J. Cell Biol. 150, 1361–1374 (2000)
Tepass, U., Theres, C. & Knust, E. crumbs encodes an EGF-like protein expressed on apical membranes of Drosophila epithelial cells and required for organization of epithelia. Cell 61, 787–799 (1990)
Pilot, F., Philippe, J. M., Lemmers, C., Chauvin, J. P. & Lecuit, T. Developmental control of nuclear morphogenesis and anchoring by charleston, identified in a functional genomic screen of Drosophila cellularisation. Development 133, 711–723 (2006)
Gabev, E., Kasianowicz, J., Abbott, T. & McLaughlin, S. Binding of neomycin to phosphatidylinositol 4,5-bisphosphate (PIP2). Biochim Biophys Acta 979, 105–112 (1989)
Zelhof, A. C. & Hardy, R. W. WASp is required for the correct temporal morphogenesis of rhabdomere microvilli. J. Cell Biol. 164, 417–426 (2004)
Formstecher, E. et al. Protein interaction mapping: a Drosophila case study. Genome Res. 15, 376–384 (2005)
Speck, O., Hughes, S. C., Noren, N. K., Kulikauskas, R. M. & Fehon, R. G. Moesin functions antagonistically to the Rho pathway to maintain epithelial integrity. Nature 421, 83–87 (2003)
Yonemura, S., Matsui, T., Tsukita, S. & Tsukita, S. Rho-dependent and-independent activation mechanisms of ezrin/radixin/moesin proteins: an essential role for polyphosphoinositides in vivo. J. Cell Sci. 115, 2569–2580 (2002)
Polesello, C., Delon, I., Valenti, P., Ferrer, P. & Payre, F. Dmoesin controls actin-based cell shape and polarity during Drosophila melanogaster oogenesis. Nature Cell Biol. 4, 782–789 (2002)
De Joussineau, C. et al. Delta-promoted filopodia mediate long-range lateral inhibition in Drosophila. Nature 426, 555–559 (2003)
Pinal, N. et al. Regulated and polarized PtdIns(3,4,5)P3 accumulation is essential for apical membrane morphogenesis in photoreceptor epithelial cells. Curr. Biol. 16, 140–149 (2006)
von Stein, W., Ramrath, A., Grimm, A., Muller-Borg, M. & Wodarz, A. Direct association of Bazooka/PAR-3 with the lipid phosphatase PTEN reveals a link between the PAR/aPKC complex and phosphoinositide signaling. Development 132, 1675–1686 (2005)
Acknowledgements
We thank all those who gave us reagents, in particular J. Serano and G. Rubin for fly strains and plasmids, A. Wodarz, A. Zelhof, R. Fehon, F. Payre, E. Suzuki, M. Peifer, H. Oda for flies, antibodies or plasmids; J. Großhans and R. Paro for reagents for synthesizing dsRNA probes for the RNAi screen. We also thank J. Knoblich for suggesting the PIPstrip experiment, members of our group for discussions, S. Kerridge and A. Lebivic for comments on the manuscript. This work was supported by the Association pour la Recherche contre le Cancer (ARC, subvention libre 5179), the CNRS, the Fondation pour la Recherche Médicale (FRM), the Fondation Schlumberger pour l'Education et la Recherche (FSER) and the EMBO Young Investigator Programme. F.P. was supported by the CNRS (bourse BDI) and by the Académie de médecine.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Reprints and permissions information is available at npg.nature.com/reprintsandpermissions. The authors declare no competing financial interests.
Supplementary information
Supplementary Methods
This file contains Supplementary Methods and legends to Supplementary Figures and Supplementary Movies. (DOC 71 kb)
Supplementary Figure S1
Phenotypes in btszRNAi and btszK13-4/btszK13-4 mutant embryos. (PDF 663 kb)
Supplementary Figure S2
Phenotypes in btsz RNAi embryos, mutants and morphants. (PDF 256 kb)
Supplementary Figure S3
Iin situ hybridization with btsz probes. (PDF 317 kb)
Supplementary Figure S4
RT-PCR in btszRNAi embryos. (PDF 47 kb)
Supplementary Movie S1
Control RNAi embryo (control 1 probe). (MOV 1780 kb)
Supplementary Movie S2
btszRNAi (probe2) embryo with a strong phenotype (red category in Fig.S2a). (MOV 1618 kb)
Supplementary Movie S3
btszRNAi (probe2) embryo with a medium phenotype (orange category in Fig.S2a). (MOV 1553 kb)
Supplementary Movie S4
btszK13-4 mat-/-, zyg+/- mutant embryo without phenotype (blue category in Fig. S2f). (MOV 1506 kb)
Supplementary Movie S5
btszK13-4 mat-/-, zyg-/- mutant embryo with a strong phenotype (red category in Fig.S2f). (MOV 1332 kb)
Supplementary Movie S6
btszK13-4 mat-/-, zyg-/- mutant embryo with a medium phenotype (red category in Fig.S2f). (MOV 1537 kb)
Supplementary Movie S7
Confocal time lapse series showing E-cad-GFP at 15-s intervals in a btszRNAi embryo. (MOV 1308 kb)
Rights and permissions
About this article
Cite this article
Pilot, F., Philippe, JM., Lemmers, C. et al. Spatial control of actin organization at adherens junctions by a synaptotagmin-like protein. Nature 442, 580–584 (2006). https://doi.org/10.1038/nature04935
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature04935
This article is cited by
-
Symbiotic microbes affect the expression of male reproductive genes in Glossina m. morsitans
BMC Microbiology (2018)
-
Guidance of subcellular tubulogenesis by actin under the control of a synaptotagmin-like protein and Moesin
Nature Communications (2014)
-
Synaptotagmin-like proteins control the formation of a single apical membrane domain in epithelial cells
Nature Cell Biology (2012)
-
Spatial regulation of Dia and Myosin-II by RhoGEF2 controls initiation of E-cadherin endocytosis during epithelial morphogenesis
Nature Cell Biology (2011)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.