Abstract
Listen to an interview with Tom Rando on the stem cells podcast
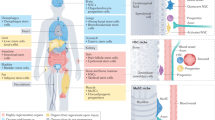
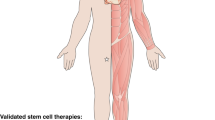
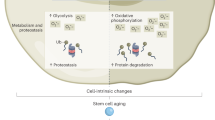
Adult stem cells reside in most mammalian tissues, but the extent to which they contribute to normal homeostasis and repair varies widely. There is an overall decline in tissue regenerative potential with age, and the question arises as to whether this is due to the intrinsic ageing of stem cells or, rather, to the impairment of stem-cell function in the aged tissue environment. Unravelling these distinct contributions to the aged phenotype will be critical to the success of any therapeutic application of stem cells in the emerging field of regenerative medicine with respect to tissue injury, degenerative diseases or normal functional declines that accompany ageing.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Phelan, J. P. & Austad, S. N. Natural selection, dietary restriction, and extended longevity. Growth Dev. Aging 53, 4–6 (1989).
Kirkwood, T. B. Understanding the odd science of aging. Cell 120, 437–447 (2005).
Medawar, P. B. An Unsolved Problem of Biology (H. K. Lewis, London, 1952).
Williams, G. C. Pleiotropy, natural selection, and the evolution of senescence. Evolution 11, 398–411 (1957).
Leroi, A. M. et al. What evidence is there for the existence of individual genes with antagonistic pleiotropic effects? Mech. Ageing Dev. 126, 421–429 (2005).
Hayflick, L. How and Why We Age (Ballantine Books, New York, 1994).
Friedman, D. B. & Johnson, T. E. A mutation in the age-1 gene in Caenorhabditis elegans lengthens life and reduces hermaphrodite fertility. Genetics 118, 75–86 (1988).
Kenyon, C., Chang, J., Gensch, E., Rudner, A. & Tabtiang, R. A C. elegans mutant that lives twice as long as wild type. Nature 366, 461–464 (1993).
Tissenbaum, H. A. & Guarente, L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature 410, 227–230 (2001).
Clancy, D. J. et al. Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science 292, 104–106 (2001).
Tatar, M. et al. A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science 292, 107–110 (2001).
Flurkey, K., Papaconstantinou, J., Miller, R. A. & Harrison, D. E. Lifespan extension and delayed immune and collagen aging in mutant mice with defects in growth hormone production. Proc. Natl Acad. Sci. USA 98, 6736–6741 (2001).
Ikeno, Y., Bronson, R. T., Hubbard, G. B., Lee, S. & Bartke, A. Delayed occurrence of fatal neoplastic diseases in ames dwarf mice: correlation to extended longevity. J. Gerontol. A Biol. Sci. Med. Sci. 58, 291–296 (2003).
Kirkwood, T. B. Evolution of ageing. Nature 270, 301–304 (1977).
Partridge, L., Gems, D. & Withers, D. J. Sex and death: what is the connection? Cell 120, 461–472 (2005).
Migliaccio, E. et al. The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature 402, 309–313 (1999).
Thorndyke, M. C., Chen, W. C., Beesley, P. W. & Patruno, M. Molecular approach to echinoderm regeneration. Microsc. Res. Tech. 55, 474–485 (2001).
Brockes, J. P. & Kumar, A. Appendage regeneration in adult vertebrates and implications for regenerative medicine. Science 310, 1919–1923 (2005).
Holstein, T. W., Hobmayer, E. & Technau, U. Cnidarians: an evolutionarily conserved model system for regeneration? Dev. Dyn. 226, 257–267 (2003).
Reddien, P. W., Bermange, A. L., Murfitt, K. J., Jennings, J. R. & Sanchez, A. A. Identification of genes needed for regeneration, stem cell function, and tissue homeostasis by systematic gene perturbation in planaria. Dev. Cell 8, 635–649 (2005).
Enver, T. et al. Cellular differentiation hierarchies in normal and culture-adapted human embryonic stem cells. Hum. Mol. Genet. 14, 3129–3140 (2005).
Park, Y. & Gerson, S. L. DNA repair defects in stem cell function and aging. Annu. Rev. Med. 56, 495–508 (2005).
Keller, G. Embryonic stem cell differentiation: emergence of a new era in biology and medicine. Genes Dev. 19, 1129–1155 (2005).
Reya, T., Morrison, S. J., Clarke, M. F. & Weissman, I. L. Stem cells, cancer, and cancer stem cells. Nature 414, 105–111 (2001).
Lombard, D. B. et al. DNA repair, genome stability, and aging. Cell 120, 497–512 (2005).
Xie, T. & Spradling, A. C. A niche maintaining germ line stem cells in the Drosophila ovary. Science 290, 328–330 (2000).
Kiger, A. A., Jones, D. L., Schulz, C., Rogers, M. B. & Fuller, M. T. Stem cell self-renewal specified by JAK–STAT activation in response to a support cell cue. Science 294, 2542–2545 (2001).
Harrison, D. E., Astle, C. M. & Doubleday, J. W. Cell lines from old immunodeficient donors give normal responses in young recipients. J. Immunol. 118, 1223–1227 (1977).
Hotta, T., Hirabayashi, N., Utsumi, M., Murate, T. & Yamada, H. Age-related changes in the function of hemopoietic stroma in mice. Exp. Hematol. 8, 933–936 (1980).
Carlson, B. M. & Faulkner, J. A. Muscle transplantation between young and old rats: age of host determines recovery. Am. J. Physiol. 256, C1262–C1266 (1989).
Conboy, I. M. et al. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 433, 760–764 (2005).
Van Zant, G. & Liang, Y. The role of stem cells in aging. Exp. Hematol. 31, 659–672 (2003).
Snyder, E. Y. & Loring, J. F. A role for stem cell biology in the physiological and pathological aspects of aging. J. Am. Geriatr. Soc. 53, S287–S291 (2005).
Lin, S. J., Defossez, P. A. & Guarente, L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science 289, 2126–2128 (2000).
Klass, M. R. Aging in the nematode Caenorhabditis elegans: major biological and environmental factors influencing life span. Mech. Ageing Dev. 6, 413–429 (1977).
Chapman, T. & Partridge, L. Female fitness in Drosophila melanogaster: an interaction between the effect of nutrition and of encounter rate with males. Proc. Biol. Sci. 263, 755–759 (1996).
Weindruch, R., Walford, R. L., Fligiel, S. & Guthrie, D. The retardation of aging in mice by dietary restriction: longevity, cancer, immunity and lifetime energy intake. J. Nutr. 116, 641–654 (1986).
Hornsby, P. J. Cellular senescence and tissue aging in vivo. J. Gerontol. A Biol. Sci. Med. Sci. 57, B251–B256 (2002).
Rubin, H. The disparity between human cell senescence in vitro and lifelong replication in vivo. Nature Biotechnol. 20, 675–681 (2002).
Parrinello, S., Coppe, J. P., Krtolica, A. & Campisi, J. Stromal–epithelial interactions in aging and cancer: senescent fibroblasts alter epithelial cell differentiation. J. Cell Sci. 118, 485–496 (2005).
Gomez, C. R., Boehmer, E. D. & Kovacs, E. J. The aging innate immune system. Curr. Opin. Immunol. 17, 457–462 (2005).
Carmel, R. Anemia and aging: an overview of clinical, diagnostic and biological issues. Blood Rev. 15, 9–18 (2001).
Sudo, K., Ema, H., Morita, Y. & Nakauchi, H. Age-associated characteristics of murine hematopoietic stem cells. J. Exp. Med. 192, 1273–1280 (2000).
Morrison, S. J., Wandycz, A. M., Akashi, K., Globerson, A. & Weissman, I. L. The aging of hematopoietic stem cells. Nature Med. 2, 1011–1016 (1996).
Harrison, D. E. & Astle, C. M. Loss of stem cell repopulating ability upon transplantation. Effects of donor age, cell number, and transplantation procedure. J. Exp. Med. 156, 1767–1779 (1982).
Harrison, D. E. Long-term erythropoietic repopulating ability of old, young, and fetal stem cells. J. Exp. Med. 157, 1496–1504 (1983).
Ogden, D. A. & Micklem, H. S. The fate of serially transplanted bone marrow cell populations from young and old donors. Transplantation 22, 287–293 (1976).
Liang, Y., Van Zant, G. & Szilvassy, S. J. Effects of aging on the homing and engraftment of murine hematopoietic stem and progenitor cells. Blood 106, 1479–1487 (2005).
Chen, J., Astle, C. M. & Harrison, D. E. Genetic regulation of primitive hematopoietic stem cell senescence. Exp. Hematol. 28, 442–450 (2000).
Van Zant, G., Scott-Micus, K., Thompson, B. P., Fleischman, R. A. & Perkins, S. Stem cell quiescence/activation is reversible by serial transplantation and is independent of stromal cell genotype in mouse aggregation chimeras. Exp. Hematol. 20, 470–475 (1992).
Rossi, D. J. et al. Cell intrinsic alterations underlie hematopoietic stem cell aging. Proc. Natl Acad. Sci. USA 102, 9194–9199 (2005).
Linton, P. J. & Dorshkind, K. Age-related changes in lymphocyte development and function. Nature Immunol. 5, 133–139 (2004).
Chargé, S. B. & Rudnicki, M. A. Cellular and molecular regulation of muscle regeneration. Physiol. Rev. 84, 209–238 (2004).
Gibson, M. C. & Schultz, E. Age-related differences in absolute numbers of skeletal muscle satellite cells. Muscle Nerve 6, 574–580 (1983).
Conboy, I. M., Conboy, M. J., Smythe, G. M. & Rando, T. A. Notch-mediated restoration of regenerative potential to aged muscle. Science 302, 1575–1577 (2003).
Brack, A. S., Bildsoe, H. & Hughes, S. M. Evidence that satellite cell decrement contributes to preferential decline in nuclear number from large fibres during murine age-related muscle atrophy. J. Cell Sci. 118, 4813–4821 (2005).
Conboy, I. M. & Rando, T. A. Aging, stem cells and tissue regeneration: lessons from muscle. Cell Cycle 4, 407–410 (2005).
Schultz, E. & Lipton, B. H. Skeletal muscle satellite cells: changes in proliferation potential as a function of age. Mech. Ageing Dev. 20, 377–383 (1982).
Bockhold, K. J., Rosenblatt, J. D. & Partridge, T. A. Aging normal and dystrophic mouse muscle: analysis of myogenicity in cultures of living single fibers. Muscle Nerve 21, 173–183 (1998).
Bortoli, S. et al. Gene expression profiling of human satellite cells during muscular aging using cDNA arrays. Gene 321, 145–154 (2003).
de Grey, A. A strategy for postponing aging indefinitely. Stud. Health Technol. Inform. 118, 209–219 (2005).
Fabrizio, P. et al. Sir2 blocks extreme life-span extension. Cell 123, 655–667 (2005).
Morgan, J. E. & Partridge, T. A. Muscle satellite cells. Int. J. Biochem. Cell Biol. 35, 1151–1156 (2003).
Sigal, S. H., Brill, S., Fiorino, A. S. & Reid, L. M. The liver as a stem cell and lineage system. Am. J. Physiol. 263, G139–G148 (1992).
Oh, S. H., Hatch, H. M. & Petersen, B. E. Hepatic oval ‘stem’ cell in liver regeneration. Semin. Cell Dev. Biol. 13, 405–409 (2002).
Gage, F. H. Stem cells of the central nervous system. Curr. Opin. Neurobiol. 8, 671–676 (1998).
Leri, A., Kajstura, J. & Anversa, P. Cardiac stem cells and mechanisms of myocardial regeneration. Physiol. Rev. 85, 1373–1416 (2005).
Ben Porath, I. & Weinberg, R. A. The signals and pathways activating cellular senescence. Int. J. Biochem. Cell Biol. 37, 961–976 (2005).
Acknowledgements
The author thanks R. Wyman (Yale University), A. Wagers (Harvard University), M. Reed (University of Washington), and C. Kuo and E. Chiao (Stanford University) for helpful discussions. This work was supported by an National Institutes of Health Director's Pioneer Award to T.A.R.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Additional information
Author Information Reprints and permissions information is available at npg.nature.com/reprintsandpermissions.
Rights and permissions
About this article
Cite this article
Rando, T. Stem cells, ageing and the quest for immortality. Nature 441, 1080–1086 (2006). https://doi.org/10.1038/nature04958
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature04958
This article is cited by
-
Stem Cell-Based Tissue Engineering Approaches for Diabetic Foot Ulcer: a Review from Mechanism to Clinical Trial
Stem Cell Reviews and Reports (2024)
-
CD133+ endothelial-like stem cells restore neovascularization and promote longevity in progeroid and naturally aged mice
Nature Aging (2023)
-
Regulation of connective tissue growth factor expression by miR-133b for the treatment of renal interstitial fibrosis in aged mice with unilateral ureteral obstruction
Stem Cell Research & Therapy (2021)
-
RETRACTED ARTICLE: Using a new HSPC senescence model in vitro to explore the mechanism of cellular memory in aging HSPCs
Stem Cell Research & Therapy (2021)
-
Photobiomodulation has rejuvenating effects on aged bone marrow mesenchymal stem cells
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.