Abstract
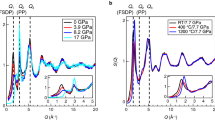
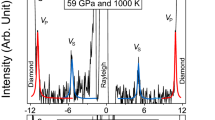
Among the group IV elements, only carbon forms stable double bonds with oxygen at ambient conditions. At variance with silica and germania, the non-molecular single-bonded crystalline form of carbon dioxide, phase V, only exists at high pressure1,2,3,4,5,6,7,8,9. The amorphous forms of silica (a-SiO2) and germania (a-GeO2) are well known at ambient conditions; however, the amorphous, non-molecular form of CO2 has so far been described only as a result of first-principles simulations9. Here we report the synthesis of an amorphous, silica-like form of carbon dioxide, a-CO2, which we call ‘a-carbonia’. The compression of the molecular phase III of CO2 between 40 and 48 GPa at room temperature initiated the transformation to the non-molecular amorphous phase. Infrared spectra measured at temperatures up to 680 K show the progressive formation of C–O single bonds and the simultaneous disappearance of all molecular signatures. Furthermore, state-of-the-art Raman and synchrotron X-ray diffraction measurements on temperature-quenched samples confirm the amorphous character of the material. Comparison with vibrational and diffraction data for a-SiO2 and a-GeO2, as well as with the structure factor calculated for the a-CO2 sample obtained by first-principles molecular dynamics9, shows that a-CO2 is structurally homologous to the other group IV dioxide glasses. We therefore conclude that the class of archetypal network-forming disordered systems, including a-SiO2, a-GeO2 and water, must be extended to include a-CO2.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Iota, V., Yoo, C. S. & Cynn, H. Quartzlike carbon dioxide: an optically nonlinear extended solid at high pressures and temperatures. Science 283, 1510–1513 (1999)
Yoo, C. S. et al. Crystal structure of carbon dioxide at high pressure: “superhard” polymeric carbon dioxide. Phys. Rev. Lett. 83, 5527–5530 (1999)
Tschauner, O., Mao, H. K. & Hemley, R. J. New transformations of CO2 at high pressures and temperatures. Phys. Rev. Lett. 87, 075701 (2001)
Santoro, M., Lin, J. F., Mao, H. K. & Hemley, R. J. In situ high P-T Raman spectroscopy and laser heating of carbon dioxide. J. Chem. Phys. 121, 2780–2787 (2004)
Dong, J., Tomfohr, J. K. & Sankey, O. F. Non-molecular carbon dioxide (CO2) solids. Science 287, 11a (2000)
Dong, J., Tomfohr, J. K. & Sankey, O. F. Rigid intertetrahedron angular interaction of nonmolecular carbon dioxide solids. Phys. Rev. B 61, 5967–5971 (2000)
Dong, J. et al. Investigation of hardness in tetrahedrally bonded nonmolecular CO2 solids by density-functional theory. Phys. Rev. B 62, 14685–14689 (2000)
Holm, B., Ahuja, R., Belonoshko, A. & Johansson, B. Theoretical investigation of high pressure phases of carbon dioxide. Phys. Rev. Lett. 85, 1258–1261 (2000)
Serra, S., Cavazzoni, C., Chiarotti, G. L., Scandolo, S. & Tosatti, E. Pressure-induced solid carbonates from molecular CO2 by computer simulation. Science 284, 788–790 (1999)
Eremets, M. I., Gavriliuk, A. G., Trojan, I. A., Dzivenko, D. A. & Boehler, R. Single-bonded cubic form of nitrogen. Nature Mater. 3, 558–563 (2004)
Goncharov, A. F., Gregoryanz, E., Mao, H. K., Liu, Z. & Hemley, R. J. Optical evidence for a nonmolecular phase of nitrogen above 150 GPa. Phys. Rev. Lett. 85, 1262–1265 (2000)
Gregoryanz, E., Goncharov, A. F., Hemley, R. J. & Mao, H. K. High pressure amorphous nitrogen. Phys. Rev. B 64, 052103 (2001)
Eremets, M. I., Hemley, R. J., Mao, H. K. & Gregoryanz, E. Semiconducting nonmolecular nitrogen up to 240 GPa and its low pressure stability. Nature 411, 170–174 (2001)
Miller, S. A. et al. Aftershocks driven by high-pressure CO2 source at depth. Nature 427, 724–727 (2004)
Kendall, J. L., Canelas, D. A., Young, J. L. & DeSimone, J. M. Polymerizations in supercritical carbon dioxide. Chem. Rev. 99, 543–563 (1999)
Schettino, V., Bini, R., Ceppatelli, M., Ciabini, L. & Citroni, M. Chemical reactions at very high pressure. Adv. Chem. Phys. 11, 105–242 (2005)
Hemley, R. J., Jephcoat, A. P., Mao, H. K., Ming, L. C. & Manghnani, M. H. Pressure-induced amorphization of crystalline silica. Nature 334, 52–54 (1988)
Williams, Q. & Jeanloz, R. Spectroscopic evidence for pressure-induced coordination changes in silicate glasses and melts. Science 239, 902–905 (1988)
Williams, Q., Hemley, R. J., Kruger, M. B. & Jeanloz, R. High-pressure infrared spectra of α-quartz, coesite, stishovite and silica glass. J. Geophys. Res. 98, 22157–22170 (1993)
Hemley, R. J., Mao, H. K., Bell, P. M. & Mysen, B. O. Raman spectroscopy of SiO2 glass at high pressure. Phys. Rev. Lett. 57, 747–750 (1986)
Durben, D. J. & Wolf, G. H. Raman spectroscopic study of the pressure-induced coordination change in GeO2 glass. Phys. Rev. B 43, 2355–2363 (1991)
Meade, C., Hemley, R. J. & Mao, H. K. High-pressure x-ray diffraction of SiO2 glass. Phys. Rev. Lett. 69, 1387–1390 (1992)
Aoki, K., Yamawaki, H., Sakashita, M., Gotoh, Y. & Takemura, K. Crystal structure of the high-pressure phase of solid CO2 . Science 263, 356–358 (1994)
Gorelli, F. A., Giordano, V. M., Salvi, P. R. & Bini, R. Linear carbon dioxide in the high-pressure high-temperature crystalline phase IV. Phys. Rev. Lett. 93, 205503 (2004)
Gorelli, F. A., Ulivi, L., Santoro, M. & Bini, R. The ɛ phase of solid oxygen: evidence of an O4 molecule lattice. Phys. Rev. Lett. 83, 4093–4096 (1999)
Hammersley, A. P., Svensson, S. O., Hanfland, M., Fitch, A. N. & Häusermann, D. Two-dimensional detector software: from real detector to idealised image or two-theta scan. High Press. Res. 14, 235–248 (1996)
Liang, Y., Miranda, C. R. & Scandolo, S. Mechanical strength and coordination defects in compressed SiO2 glass. Phys. Rev. Lett. (submitted)
Acknowledgements
We thank F. Sette and M. Mezouar for discussions and the ESRF for provision of beamtime at ID27. S.S. thanks Y. Liang for preparing the molecular dynamics configurations. This work was supported by the European Community and the Ente Cassa di Risparmio di Firenze.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
Reprints and permissions information is available at npg.nature.com/reprintsandpermissions. The authors declare no competing financial interests.
Supplementary information
Supplementary Figures
This file shows the procedure used for the x-ray diffraction data processing. The figure shows the diffracted total intensity I(Q), the background intensity Ib(Q) and the sample diffracted intensity, given by the difference between the two. The legend describes how to obtain the S(Q) from the sample diffracted intensity. (PDF 35 kb)
Rights and permissions
About this article
Cite this article
Santoro, M., Gorelli, F., Bini, R. et al. Amorphous silica-like carbon dioxide. Nature 441, 857–860 (2006). https://doi.org/10.1038/nature04879
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature04879
This article is cited by
-
Structure determination of ζ-N2 from single-crystal X-ray diffraction and theoretical suggestion for the formation of amorphous nitrogen
Nature Communications (2023)
-
High pressure synthesis of phosphine from the elements and the discovery of the missing (PH3)2H2 tile
Nature Communications (2020)
-
CO3+1 network formation in ultra-high pressure carbonate liquids
Scientific Reports (2019)
-
Crystalline polymeric carbon dioxide stable at megabar pressures
Nature Communications (2018)
-
Pressure-induced Transformations of Dense Carbonyl Sulfide to Singly Bonded Amorphous Metallic Solid
Scientific Reports (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.