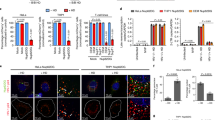
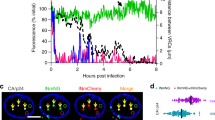
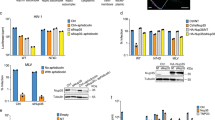
Abstract
Primate lentiviruses such as human immunodeficiency type 1 (HIV-1) have the capacity to infect non-dividing cells such as tissue macrophages1. In the process, viral complementary DNA traverses the nuclear envelope to integrate within chromatin2. Given the intimate association between chromatin and the nuclear envelope3, we examined whether HIV-1 appropriates nuclear envelope components during infection. Here we show that emerin, an integral inner-nuclear-envelope protein, is necessary for HIV-1 infection. Infection of primary macrophages lacking emerin was abortive in that viral cDNA localized to the nucleus but integration into chromatin was inefficient, and conversion of viral cDNA to non-functional episomal cDNA increased. HIV-1 cDNA associated with emerin in vivo, and the interaction of viral cDNA with chromatin was dependent on emerin. Barrier-to-autointegration factor (BAF), the LEM (LAP, emerin, MAN) binding partner of emerin, was required for the association of viral cDNA with emerin and for the ability of emerin to support virus infection. Therefore emerin, which bridges the interface between the inner nuclear envelope and chromatin, may be necessary for chromatin engagement by viral cDNA before integration.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Stevenson, M. HIV-1 pathogenesis. Nature Med. 9, 853–860 (2003)
Greene, W. C. & Peterlin, B. M. Charting HIV's remarkable voyage through the cell: Basic science as a passport to future therapy. Nature Med. 8, 673–680 (2002)
Gruenbaum, Y., Margalit, A., Goldman, R. D., Shumaker, D. K. & Wilson, K. L. The nuclear lamina comes of age. Nature Rev. Mol. Cell Biol. 6, 21–31 (2005)
Zastrow, M. S., Vlcek, S. & Wilson, K. L. Proteins that bind A-type lamins: Integrating isolated clues. J. Cell Sci. 117, 979–987 (2004)
Song, E. et al. Sustained small interfering RNA-mediated human immunodeficiency virus type 1 inhibition in primary macrophages. J. Virol. 77, 7174–7181 (2003)
Zielske, S. P. & Stevenson, M. Importin 7 may be dispensable for human immunodeficiency virus type 1 and simian immunodeficiency virus infection of primary macrophages. J. Virol. 79, 11541–11546 (2005)
Farnet, C. M. & Bushman, F. D. HIV-1 cDNA integration: requirement of HMG I(Y) protein for function of preintegration complexes in vitro. Cell 88, 483–492 (1997)
Li, L. et al. Retroviral cDNA integration: stimulation by HMG I family proteins. J. Virol. 74, 10965–10974 (2000)
Beitzel, B. & Bushman, F. Construction and analysis of cells lacking the HMGA gene family. Nucleic Acids Res. 31, 5025–5032 (2003)
Lewis, P. F. & Emerman, M. Passage through mitosis is required for oncoretroviruses but not for the human immunodeficiency virus. J. Virol. 68, 510–516 (1994)
Suzuki, Y., Yang, H. & Craigie, R. LAP2α and BAF collaborate to organize the Moloney murine leukemia virus preintegration complex. EMBO J. 23, 4670–4678 (2004)
Swingler, S. et al. HIV-1 Nef mediates lymphocyte chemotaxis and activation by infected macrophages. Nature Med. 5, 997–1003 (1999)
Mansharamani, M. et al. Barrier-to-autointegration factor BAF binds p55 Gag and matrix and is a host component of human immunodeficiency virus type 1 virions. J. Virol. 77, 13084–13092 (2003)
Lee, M. S. & Craigie, R. A previously unidentified host protein protects retroviral DNA from autointegration. Proc. Natl Acad. Sci. USA 95, 1528–1533 (1998)
Segura-Totten, M. & Wilson, K. L. BAF: roles in chromatin, nuclear structure and retrovirus integration. Trends Cell Biol. 14, 261–266 (2004)
Miccoli, L. et al. Selective interactions of human kin17 and RPA proteins with chromatin and the nuclear matrix in a DNA damage- and cell cycle-regulated manner. Nucleic Acids Res. 31, 4162–4175 (2003)
Lu, R. et al. Class II integrase mutants with changes in putative nuclear localization signals are primarily blocked at a postnuclear entry step of human immunodeficiency virus type 1 replication. J. Virol. 78, 12735–12746 (2004)
Lau, A., Kanaar, R., Jackson, S. P. & O'Connor, M. J. Suppression of retroviral infection by the RAD52 DNA repair protein. EMBO J. 23, 3421–3429 (2004)
Lin, C. W. & Engelman, A. The barrier-to-autointegration factor is a component of functional human immunodeficiency virus type 1 preintegration complexes. J. Virol. 77, 5030–5036 (2003)
Stevenson, M. et al. Integration is not necessary for expression of human immunodeficiency virus type 1 protein products. J. Virol. 64, 2421–2425 (1990)
Cherepanov, P., Ambrosio, A. L., Rahman, S., Ellenberger, T. & Engelman, A. Structural basis for the recognition between HIV-1 integrase and transcriptional coactivator p75. Proc. Natl Acad. Sci. USA 102, 17308–17313 (2005)
Segura-Totten, M., Kowalski, A. K., Craigie, R. & Wilson, K. L. Barrier-to-autointegration factor: Major roles in chromatin decondensation and nuclear assembly. J. Cell Biol. 158, 475–485 (2002)
Lee, K. K. et al. Distinct functional domains in emerin bind lamin A and DNA-bridging protein BAF. J. Cell Sci. 114, 4567–4573 (2001)
Wiskerchen, M. & Muesing, M. A. Human immunodeficiency virus type 1 interase: Effects of mutations on viral ability to integrate, direct viral gene expression from unintegrated viral DNA templates, and sustain viral propagation in primary cells. J. Virol. 69, 376–386 (1995)
Hazuda, D. J. et al. Inhibitors of strand transfer that prevent integration and inhibit HIV-1 replication in cells. Science 287, 646–650 (2000)
Emery, A. E. The muscular dystrophies. Lancet 359, 687–695 (2002)
Jacque, J.-M., Triques, K. & Stevenson, M. Modulation of HIV-1 replication by RNAi. Nature 418, 435–438 (2002)
Acknowledgements
We thank A. Dauphin and J. Zhou for research support, B. Mellor for preparation of the figures, K. Departie and L. Smith for manuscript preparation, and A. Engelman for providing HIV-1 integrase mutants. The University of Massachusetts Center for AIDS Research and the AIDS Research Reference Reagent Program, Division of AIDS, NIAID, National Institutes of Health (NIH), provided assay support and reagents. This study was supported by grants from the NIH to M.S.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Reprints and permissions information is available at npg.nature.com/reprintsandpermissions. The authors declare no competing financial interests.
Supplementary information
Supplementary Figures
This file contains Supplementary Figures 1 and 2. (PDF 264 kb)
Supplementary Methods
This file contains additional information on the methods used in this study, including Cells and siRNA transfections, PCR analysis and Western blotting. This file also contains additional references. (DOC 54 kb)
Rights and permissions
About this article
Cite this article
Jacque, JM., Stevenson, M. The inner-nuclear-envelope protein emerin regulates HIV-1 infectivity. Nature 441, 641–645 (2006). https://doi.org/10.1038/nature04682
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature04682
This article is cited by
-
BANF1 promotes glutamate-induced apoptosis of HT-22 hippocampal neurons
Molecular Biology Reports (2023)
-
Vpu modulates DNA repair to suppress innate sensing and hyper-integration of HIV-1
Nature Microbiology (2020)
-
The macrophage: a therapeutic target in HIV-1 infection
Molecular and Cellular Therapies (2014)
-
Quantitative analysis of the time-course of viral DNA forms during the HIV-1 life cycle
Retrovirology (2013)
-
Molecular genetic analysis of VRK1 in mammary epithelial cells: depletion slows proliferation in vitro and tumor growth and metastasis in vivo
Oncogenesis (2013)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.