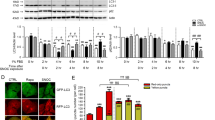
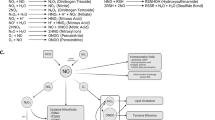
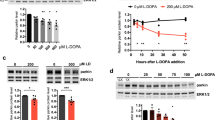
Abstract
Stress proteins located in the cytosol or endoplasmic reticulum (ER) maintain cell homeostasis and afford tolerance to severe insults1,2,3. In neurodegenerative diseases, several chaperones ameliorate the accumulation of misfolded proteins triggered by oxidative or nitrosative stress, or of mutated gene products4,5. Although severe ER stress can induce apoptosis2,6, the ER withstands relatively mild insults through the expression of stress proteins or chaperones such as glucose-regulated protein (GRP) and protein-disulphide isomerase (PDI), which assist in the maturation and transport of unfolded secretory proteins. PDI catalyses thiol–disulphide exchange, thus facilitating disulphide bond formation and rearrangement reactions7,8,9,10. PDI has two domains that function as independent active sites with homology to the small, redox-active protein thioredoxin7,8. During neurodegenerative disorders and cerebral ischaemia, the accumulation of immature and denatured proteins results in ER dysfunction11, but the upregulation of PDI represents an adaptive response to protect neuronal cells12,13,14. Here we show, in brains manifesting sporadic Parkinson's or Alzheimer's disease, that PDI is S-nitrosylated, a reaction transferring a nitric oxide (NO) group to a critical cysteine thiol to affect protein function15,16,17,18. NO-induced S-nitrosylation of PDI inhibits its enzymatic activity, leads to the accumulation of polyubiquitinated proteins, and activates the unfolded protein response. S-Nitrosylation also abrogates PDI-mediated attenuation of neuronal cell death triggered by ER stress, misfolded proteins or proteasome inhibition. Thus, PDI prevents neurotoxicity associated with ER stress and protein misfolding, but NO blocks this protective effect in neurodegenerative disorders through the S-nitrosylation of PDI.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ellgaard, L., Molinari, M. & Helenius, A. Setting the standards: quality control in the secretory pathway. Science 286, 1882–1888 (1999)
Kaufman, R. J. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev. 13, 1211–1233 (1999)
Patil, C. & Walter, P. Intracellular signaling from the endoplasmic reticulum to the nucleus: the unfolded protein response in yeast and mammals. Curr. Opin. Cell Biol. 13, 349–355 (2001)
Rao, R. V. & Bredesen, D. E. Misfolded proteins, endoplasmic reticulum stress and neurodegeneration. Curr. Opin. Cell Biol. 16, 653–662 (2004)
Haynes, C. M., Titus, E. A. & Cooper, A. A. Degradation of misfolded proteins prevents ER-derived oxidative stress and cell death. Mol. Cell 15, 767–776 (2004)
Imai, Y. et al. An unfolded putative transmembrane polypeptide, which can lead to endoplasmic reticulum stress, is a substrate of Parkin. Cell 105, 891–902 (2001)
Edman, J. C., Ellis, L., Blacher, R. W., Roth, R. A. & Rutter, W. J. Sequence of protein disulphide isomerase and implications of its relationship to thioredoxin. Nature 317, 267–270 (1985)
Vuori, K., Myllyla, R., Pihlajaniemi, T. & Kivirikko, K. I. Expression and site-directed mutagenesis of human protein disulfide isomerase in Escherichia coli. This multifunctional polypeptide has two independently acting catalytic sites for the isomerase activity. J. Biol. Chem. 267, 7211–7214 (1992)
Song, J. L. & Wang, C. C. Chaperone-like activity of protein disulfide-isomerase in the refolding of rhodanese. Eur. J. Biochem. 231, 312–316 (1995)
Gilbert, H. F. Protein disulfide isomerase. Methods Enzymol. 290, 26–50 (1998)
Hu, B. R., Martone, M. E., Jones, Y. Z. & Liu, C. L. Protein aggregation after transient cerebral ischemia. J. Neurosci. 20, 3191–3199 (2000)
Ko, H. S., Uehara, T. & Nomura, Y. Role of ubiquilin associated with protein-disulfide isomerase in the endoplasmic reticulum in stress-induced apoptotic cell death. J. Biol. Chem. 277, 35386–35392 (2002)
Tanaka, S., Uehara, T. & Nomura, Y. Up-regulation of protein-disulfide isomerase in response to hypoxia/brain ischemia and its protective effect against apoptotic cell death. J. Biol. Chem. 275, 10388–10393 (2000)
Conn, K. J. et al. Identification of the protein disulfide isomerase family member PDIp in experimental Parkinson's disease and Lewy body pathology. Brain Res. 1022, 164–172 (2004)
Lipton, S. A. et al. A redox-based mechanism for the neuroprotective and neurodestructive effects of nitric oxide and related nitroso-compounds. Nature 364, 626–632 (1993)
Stamler, J. S. Redox signaling: nitrosylation and related target interactions of nitric oxide. Cell 78, 931–936 (1994)
Haendeler, J. et al. Redox regulatory and anti-apoptotic functions of thioredoxin depend on S-nitrosylation at cysteine 69. Nature Cell Biol. 4, 743–749 (2002)
Sliskovic, I., Raturi, A. & Mutus, B. Characterization of the S-denitrosation activity of protein disulfide isomerase. J. Biol. Chem. 280, 8733–8741 (2005)
Gu, Z. et al. S-Nitrosylation of matrix metalloproteinases: signaling pathway to neuronal cell death. Science 297, 1186–1190 (2002)
Yao, D. et al. Nitrosative stress linked to sporadic Parkinson's disease: S-nitrosylation of parkin regulates its E3 ubiquitin ligase activity. Proc. Natl Acad. Sci. USA 101, 10810–10814 (2004)
Jaffrey, S. R., Erdjument-Bromage, H., Ferris, C. D., Tempst, P. & Snyder, S. H. Protein S-nitrosylation: a physiological signal for neuronal nitric oxide. Nature Cell Biol. 3, 193–197 (2001)
Betarbet, R. et al. Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nature Neurosci. 3, 1301–1306 (2000)
Nishikawa, A. et al. Novel function of PS2V: change in conformation of tau proteins. Biochem. Biophys. Res. Commun. 318, 435–438 (2004)
Bonfoco, E., Krainc, D., Ankarcrona, M., Nicotera, P. & Lipton, S. A. Apoptosis and necrosis: two distinct events induced, respectively, by mild and intense insults with N-methyl-d-aspartate or nitric oxide/superoxide in cortical cell cultures. Proc. Natl Acad. Sci. USA 92, 7162–7166 (1995)
Dawson, V. L., Dawson, T. M., London, E. D., Bredt, D. S. & Snyder, S. H. Nitric oxide mediates glutamate neurotoxicity in primary cortical cultures. Proc. Natl Acad. Sci. USA 88, 6368–6371 (1991)
Lipton, S. A. & Rosenberg, P. A. Excitatory amino acids as a final common pathway for neurologic disorders. N. Engl. J. Med. 330, 613–622 (1994)
Hara, M. R. et al. S-nitrosylated GAPDH initiates apoptotic cell death by nuclear translocation following Siah1 binding. Nature Cell Biol. 7, 665–674 (2005)
Murakami, T. et al. Pael-R is accumulated in Lewy bodies of Parkinson's disease. Ann. Neurol. 55, 439–442 (2004)
Ryu, E. J. et al. Endoplasmic reticulum stress and the unfolded protein response in cellular models of Parkinson's disease. J. Neurosci. 22, 10690–10698 (2002)
Chung, K. K. et al. S-Nitrosylation of parkin regulates ubiquitination and compromises parkin's protective function. Science 304, 1328–1331 (2004)
Acknowledgements
We thank X. Fang for preparation of cerebrocortical cultures, T. William for technical assistance with the analysis of mass spectra, and R. Takahashi for the Pael receptor construct. T.U. was supported in part by the Mitsubishi Pharma Research Foundation and a Grant-in-Aid from the Ministry of Education, Culture, Sports and Technology of Japan. S.A.L. was supported in part by grants from the NIH, the American Parkinson's Disease Association, San Diego Chapter, and an Ellison Senior Scholars Award in Aging. Author Contributions T.U. and T.N. performed most of the experiments, contributing equally to the work, and helped to write the manuscript. D.Y., Z.Q.S. and Z.G. provided the biochemical data, and also contributed equally to the work. Y.M. analyzed the mass spectrometry data. E.M. provided the human subjects, and Y.N. provided constructs and advice. S.A.L., the senior author, designed the project, helped to analyse the data, wrote the manuscript and provided the financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Reprints and permissions information is available at npg.nature.com/reprintsandpermissions. The authors declare that they have no competing financial interests.
Supplementary information
Supplementary Figure 1
Supplementary Figure 1 a and b detail S-Nitrosylation of PDI in cells. (PDF 513 kb)
Supplementary Figure 2
LC-MS/MS analysis of PDI showing modified cysteine thiol groups in the C-terminal CGHC motif. (PDF 106 kb)
Supplementary Figure 3
S-Nitrosylation of PDI in human brains. (PDF 173 kb)
Supplementary Figure 4
Effect of oxidative and nitrosative stress on PDI activity. (PDF 127 kb)
Supplementary Figure 5
Colocalization of overexpressed PDI protein with the ER marker Calreticulin in SH-SY5Y cells. (PDF 384 kb)
Supplementary Figure 6
Effect of endogenous NO and transduced PDI on cell survival. (PDF 213 kb)
Supplementary Figure 7
Overexpression of PDI by adenoviral infection. (PDF 669 kb)
Supplementary Table 1
List of human brain subjects used in this study. (PDF 175 kb)
Supplementary Notes
This file contains Supplementary Methods and additional references. (DOC 68 kb)
Rights and permissions
About this article
Cite this article
Uehara, T., Nakamura, T., Yao, D. et al. S-Nitrosylated protein-disulphide isomerase links protein misfolding to neurodegeneration. Nature 441, 513–517 (2006). https://doi.org/10.1038/nature04782
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature04782
This article is cited by
-
Dnmt3b ablation affects fracture repair process by regulating apoptosis
BMC Musculoskeletal Disorders (2024)
-
Protein disulfide isomerase A1 as a novel redox sensor in VEGFR2 signaling and angiogenesis
Angiogenesis (2023)
-
Myofilament dysfunction in diastolic heart failure
Heart Failure Reviews (2023)
-
Targeted protein S-nitrosylation of ACE2 inhibits SARS-CoV-2 infection
Nature Chemical Biology (2023)
-
Pivotal role for S-nitrosylation of DNA methyltransferase 3B in epigenetic regulation of tumorigenesis
Nature Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.