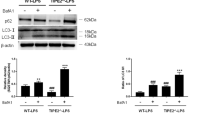
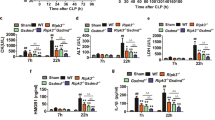
Abstract
Caspases function in both apoptosis and inflammatory cytokine processing and thereby have a role in resistance to sepsis1. Here we describe a novel role for a caspase in dampening responses to bacterial infection. We show that in mice, gene-targeted deletion of caspase-12 renders animals resistant to peritonitis and septic shock. The resulting survival advantage was conferred by the ability of the caspase-12-deficient mice to clear bacterial infection more efficiently than wild-type littermates. Caspase-12 dampened the production of the pro-inflammatory cytokines interleukin (IL)-1β, IL-18 (interferon (IFN)-γ inducing factor) and IFN-γ, but not tumour-necrosis factor-α and IL-6, in response to various bacterial components that stimulate Toll-like receptor and NOD pathways. The IFN-γ pathway was crucial in mediating survival of septic caspase-12-deficient mice, because administration of neutralizing antibodies to IFN-γ receptors ablated the survival advantage that otherwise occurred in these animals. Mechanistically, caspase-12 associated with caspase-1 and inhibited its activity. Notably, the protease function of caspase-12 was not necessary for this effect, as the catalytically inactive caspase-12 mutant Cys299Ala also inhibited caspase-1 and IL-1β production to the same extent as wild-type caspase-12. In this regard, caspase-12 seems to be the cFLIP counterpart for regulating the inflammatory branch of the caspase cascade. In mice, caspase-12 deficiency confers resistance to sepsis and its presence exerts a dominant-negative suppressive effect on caspase-1, resulting in enhanced vulnerability to bacterial infection and septic mortality.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
29 May 2013
Nature 440, 1064–1068 (2006); doi:10.1038/nature04656 Owing to an error in the production process, some details were omitted from the advance online publication version of this Corrigendum: this is the complete version. When our Letter was under consideration at Nature, we originally showed co-immunoprecipitation between caspase-1 and wild-type caspase-12 or catalytically inactive caspase-12 (C299A) as part of Fig.
References
Hotchkiss, R. S. et al. Caspase inhibitors improve survival in sepsis: a critical role of the lymphocyte. Nature Immunol. 1, 496–501 (2000)
Li, P. et al. Mice deficient in IL-1β-converting enzyme are defective in production of mature IL-1β and resistant to endotoxic shock. Cell 80, 401–411 (1995)
Kuida, K. et al. Altered cytokine export and apoptosis in mice deficient in interlukin-1β converting enzyme. Science 267, 2000–2003 (1995)
Wang, S. et al. Murine caspase-11, an ICE-interacting protease, is essential for the activation of ICE. Cell 92, 501–509 (1998)
Saleh, M. et al. Differential modulation of endotoxin responsiveness by human caspase-12 polymorphisms. Nature 429, 75–79 (2004)
Zantl, N. et al. Essential role of gamma interferon in survival of colon ascendens stent peritonitis, a novel murine model of abdominal sepsis. Infect. Immun. 66, 2300–2309 (1998)
Hotchkiss, R. S. et al. Adoptive transfer of apoptotic splenocytes worsens survival, whereas adoptive transfer of necrotic splenocytes improves survival in sepsis. Proc. Natl Acad. Sci. USA 100, 6724–6729 (2003)
Prass, K. et al. Stroke-induced immunodeficiency promotes spontaneous bacterial infections and is mediated by sympathetic activation reversal by poststroke T helper cell type 1-like immunostimulation. J. Exp. Med. 198, 725–736 (2003)
Rasper, D. M. et al. Cell death attenuation by ‘Usurpin’, a mammalian DED-caspase homologue that precludes caspase-8 recruitment and activation by the CD-95 (Fas, APO-1) receptor complex. Cell Death Differ. 5, 271–288 (1998)
Irmler, M. et al. Inhibition of death receptor signals by cellular FLIP. Nature 388, 190–195 (1997)
Martinon, F., Burns, K. & Tschopp, J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-β. Mol. Cell 10, 417–426 (2002)
Joshi, V. D., Kalvakolanu, D. V., Hebel, J. R., Hasday, J. D. & Cross, A. S. Role of caspase 1 in murine antibacterial host defenses and lethal endotoxemia. Infect. Immun. 70, 6896–6903 (2002)
Watanabe, T., Kitani, A., Murray, P. J. & Strober, W. NOD2 is a negative regulator of Toll-like receptor 2-mediated T helper type 1 responses. Nature Immunol. 5, 800–808 (2004)
Kobayashi, K. S. et al. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science 307, 731–734 (2005)
Nakagawa, T. et al. Caspase-12 mediates endoplasmic reticulum-specific apoptosis and cytotoxicity by amyloid-β. Nature 403, 98–103 (2000)
Kalai, M. et al. Regulation of the expression and processing of caspase-12. J. Cell Biol. 162, 457–467 (2003)
Obeng, E. A. & Boise, L. H. Caspase-12 and caspase-4 are not required for caspase-dependent endoplasmic reticulum stress-induced apoptosis. J. Biol. Chem. 280, 29578–29587 (2005)
Hitomi, J. et al. Involvement of caspase-4 in endoplasmic reticulum stress-induced apoptosis and Aβ-induced cell death. J. Cell Biol. 165, 347–356 (2004)
Acknowledgements
M.S. is supported by a CIHR post-doctoral fellowship. D.R.G. is supported by grants from the US NIH. We thank S. Granger and A. Coddington for help with the Bio-plex system and C. Bonzon for help with MEF preparation. Author Contributions D.R.G. and D.W.N. share senior authorship.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
D.W.N. is Vice-President of Merck Research Laboratories. It is highly unlikely, but possible, that D.W.N. or the company would gain or lose financially through publication of this paper.
Supplementary information
Supplementary Figures
Supplementary Figures nature04656-s1.ppt This file contains Supplementary Figures 1–6. (PPT 5452 kb)
Supplementary Figure Legends
This file contains text to accompany the above Supplementary Figures. (DOC 23 kb)
Supplementary Methods
This file contains additional details of the methods used in this study. (DOC 60 kb)
Rights and permissions
About this article
Cite this article
Saleh, M., Mathison, J., Wolinski, M. et al. Enhanced bacterial clearance and sepsis resistance in caspase-12-deficient mice. Nature 440, 1064–1068 (2006). https://doi.org/10.1038/nature04656
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature04656
This article is cited by
-
Apoptotic caspase-7 activation inhibits non-canonical pyroptosis by GSDMB cleavage
Cell Death & Differentiation (2023)
-
Mechanism of inositol-requiring enzyme 1-alpha inhibition in endoplasmic reticulum stress and apoptosis in ovarian cancer cells
Journal of Cell Communication and Signaling (2020)
-
Caspases in metabolic disease and their therapeutic potential
Cell Death & Differentiation (2018)
-
BH3-only proteins: the thorny end of the ER stress response
Cell Death & Disease (2017)
-
Cold-inducible RNA-binding protein (CIRP) causes sepsis-associated acute lung injury via induction of endoplasmic reticulum stress
Scientific Reports (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.