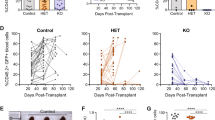
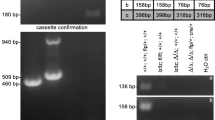
Abstract
Chromosomal translocations involving the immunoglobulin switch region are a hallmark feature of B-cell malignancies1. However, little is known about the molecular mechanism by which primary B cells acquire or guard against these lesions. Here we find that translocations between c-myc and the IgH locus (Igh) are induced in primary B cells within hours of expression of the catalytically active form of activation-induced cytidine deaminase (AID), an enzyme that deaminates cytosine to produce uracil in DNA2,3. Translocation also requires uracil DNA glycosylase (UNG), which removes uracil from DNA to create abasic sites that are then processed to double-strand breaks4,5. The pathway that mediates aberrant joining of c-myc and Igh differs from intrachromosomal repair during immunoglobulin class switch recombination in that it does not require histone H2AX6, p53 binding protein 1 (53BP1)7,8 or the non-homologous end-joining protein Ku809. In addition, translocations are inhibited by the tumour suppressors ATM, Nbs1, p19 (Arf) and p53, which is consistent with activation of DNA damage- and oncogenic stress-induced checkpoints during physiological class switching. Finally, we demonstrate that accumulation of AID-dependent, IgH-associated chromosomal lesions is not sufficient to enhance c-myc–Igh translocations. Our findings reveal a pathway for surveillance and protection against AID-dependent DNA damage, leading to chromosomal translocations.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Kuppers, R. & Dalla-Favera, R. Mechanisms of chromosomal translocations in B cell lymphomas. Oncogene 20, 5580–5594 (2001)
Muramatsu, M. et al. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell 102, 553–563 (2000)
Petersen-Mahrt, S. K., Harris, R. S. & Neuberger, M. S. AID mutates E. coli suggesting a DNA deamination mechanism for antibody diversification. Nature 418, 99–103 (2002)
Rada, C. et al. Immunoglobulin isotype switching is inhibited and somatic hypermutation perturbed in UNG-deficient mice. Curr. Biol. 12, 1748–1755 (2002)
Schrader, C. E., Linehan, E. K., Mochegova, S. N., Woodland, R. T. & Stavnezer, J. Inducible DNA breaks in IgS regions are dependent on AID and UNG. J. Exp. Med. 202, 561–568 (2005)
Petersen, S. et al. AID is required to initiate Nbs1/γ-H2AX focus formation and mutations at sites of class switching. Nature 414, 660–665 (2001)
Ward, I. M. et al. 53BP1 is required for class switch recombination. J. Cell Biol. 165, 459–464 (2004)
Manis, J. P. et al. 53BP1 links DNA damage-response pathways to immunoglobulin heavy chain class-switch recombination. Nature Immunol. 5, 481–487 (2004)
Casellas, R. et al. Ku80 is required for immunoglobulin isotype switching. EMBO J. 17, 2404–2411 (1998)
Revy, P. et al. Activation-induced cytidine deaminase (AID) deficiency causes the autosomal recessive form of the Hyper-IgM syndrome (HIGM2). Cell 102, 565–575 (2000)
Potter, M. & Wiener, F. Plasmacytomagenesis in mice: model of neoplastic development dependent upon chromosomal translocations. Carcinogenesis 13, 1681–1697 (1992)
Ramiro, A. R. et al. AID is required for c-myc/IgH chromosome translocations in vivo. Cell 118, 431–438 (2004)
Unniraman, S., Zhou, S. & Schatz, D. G. Identification of an AID-independent pathway for chromosomal translocations between the IgH switch region and Myc. Nature Immunol. 5, 1117–1123 (2004)
Imai, K. et al. Human uracil-DNA glycosylase deficiency associated with profoundly impaired immunoglobulin class-switch recombination. Nature Immunol. 4, 1023–1028 (2003)
Endres, M. et al. Increased postischemic brain injury in mice deficient in uracil-DNA glycosylase. J. Clin. Invest. 113, 1711–1721 (2004)
Manis, J. P. et al. Ku70 is required for late B cell development and immunoglobulin heavy chain class switching. J. Exp. Med. 187, 2081–2089 (1998)
Manis, J. P., Dudley, D., Kaylor, L. & Alt, F. W. IgH class switch recombination to IgG1 in DNA-PKcs-deficient B cells. Immunity 16, 607–617 (2002)
Bosma, G. C. et al. DNA-dependent protein kinase activity is not required for immunoglobulin class switching. J. Exp. Med. 196, 1483–1495 (2002)
Roschke, V., Kopantzev, E., Dertzbaugh, M. & Rudikoff, S. Chromosomal translocations deregulating c-myc are associated with normal immune responses. Oncogene 14, 3011–3016 (1997)
Reina-San-Martin, B., Chen, H. T., Nussenzweig, A. & Nussenzweig, M. C. ATM is required for efficient recombination between immunoglobulin switch regions. J. Exp. Med. 200, 1103–1110 (2004)
Lumsden, J. M. et al. Immunoglobulin class switch recombination is impaired in Atm-deficient mice. J. Exp. Med. 200, 1111–1121 (2004)
Reina-San-Martin, B., Nussenzweig, M. C., Nussenzweig, A. & Difilippantonio, S. Genomic instability, endoreduplication, and diminished Ig class-switch recombination in B cells lacking Nbs1. Proc. Natl Acad. Sci. USA 102, 1590–1595 (2005)
Kracker, S. et al. Nibrin functions in Ig class-switch recombination. Proc. Natl Acad. Sci. USA 102, 1584–1589 (2005)
Reina-San-Martin, B. et al. H2AX is required for recombination between immunoglobulin switch regions but not for intra-switch region recombination or somatic hypermutation. J. Exp. Med. 197, 1767–1778 (2003)
Difilippantonio, S. et al. Role of Nbs1 in the activation of the Atm kinase revealed in humanized mouse models. Nature Cell Biol. 7, 675–685 (2005)
Lowe, S. W. & Sherr, C. J. Tumor suppression by Ink4a-Arf: progress and puzzles. Curr. Opin. Genet. Dev. 13, 77–83 (2003)
Difilippantonio, M. J. et al. Evidence for replicative repair of DNA double-strand breaks leading to oncogenic translocation and gene amplification. J. Exp. Med. 196, 469–480 (2002)
Ward, I. M. et al. 53BP1 cooperates with p53 and functions as a haploinsufficient tumour suppressor in mice. Mol. Cell. Biol. 25, 10079–10086 (2005)
Bassing, C. H. et al. Histone H2AX: a dosage-dependent suppressor of oncogenic translocations and tumors. Cell 114, 359–370 (2003)
Kovalchuk, A. L. et al. IL-6 transgenic mouse model for extraosseous plasmacytoma. Proc. Natl Acad. Sci. USA 99, 1509–1514 (2002)
Acknowledgements
We thank T. Honjo for Aid-/- mice, M. Bosma for DNA-PKcs-/- mice, R. Jaenisch for Ung-/- mice, A. Singer and E. Besmer for suggestions, K. Velinzon for flow cytometry, and L. Stapelton for painting probes. A.N. was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research and a grant from the AT Childrens Project. S.W.L. was supported by grants from the National Cancer Institute. A.R.R. is a Ramon y Cajal investigator from Ministerio de Educacion y Ciencia, Spain. M.C.N. was supported by grants from the NIH and the Leukemia Society. M.C.N. is a Howard Hughes Institute Investigator. Author Contributions The last two authors (A.N. and M.C.N.) contributed equally to this work.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
Reprints and permissions information is available at npg.nature.com/reprintsandpermissions. The authors declare no competing financial interests.
Supplementary information
Supplementary Notes
This file contains Supplementary Figures 1-5, Supplementary Methods and Supplementary Table 1. (PDF 7313 kb)
Rights and permissions
About this article
Cite this article
Ramiro, A., Jankovic, M., Callen, E. et al. Role of genomic instability and p53 in AID-induced c-myc–Igh translocations. Nature 440, 105–109 (2006). https://doi.org/10.1038/nature04495
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature04495
This article is cited by
-
Functions and consequences of AID/APOBEC-mediated DNA and RNA deamination
Nature Reviews Genetics (2022)
-
Citrus Mealybug Performance and Plant Strata Preference on Different Coffee Varieties
Neotropical Entomology (2021)
-
Activation-induced cytidine deaminase overexpression in double-hit lymphoma: potential target for novel anticancer therapy
Scientific Reports (2020)
-
Repair of G1 induced DNA double-strand breaks in S-G2/M by alternative NHEJ
Nature Communications (2020)
-
The KT Jeang Prize 2019: Reuben S. Harris
Retrovirology (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.