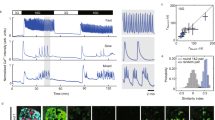
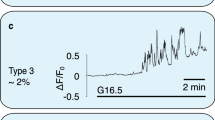
Abstract
Cyclic AMP is a ubiquitous second messenger that transduces signals from a variety of cell surface receptors to regulate diverse cellular functions, including secretion, metabolism and gene transcription. In pancreatic β-cells, cAMP potentiates Ca2+-dependent exocytosis1,2,3 and mediates the stimulation of insulin release exerted by the hormones glucagon and glucagon-like peptide-1 (GLP-1) (refs 4, 5–6). Whereas Ca2+ signals have been extensively characterized and shown to involve oscillations important for the temporal control of insulin secretion4,7,8, the kinetics of receptor-triggered cAMP signals is unknown. Here we introduce a new ratiometric evanescent-wave-microscopy approach to measure cAMP concentration beneath the plasma membrane, and show that insulin-secreting β-cells respond to glucagon and GLP-1 with marked cAMP oscillations. Simultaneous measurements of intracellular Ca2+ concentration revealed that the two messengers are interlinked and reinforce each other. Moreover, cAMP oscillations are capable of inducing rapid on–off Ca2+ responses, but only sustained elevation of cAMP concentration induces nuclear translocation of the catalytic subunit of the cAMP-dependent protein kinase. Our results establish a new signalling mode for cAMP and indicate that temporal encoding of cAMP signals might constitute a basis for differential regulation of downstream cellular targets.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Prentki, M. & Matschinsky, F. M. Ca2+, cAMP, and phospholipid-derived messengers in coupling mechanisms of insulin secretion. Physiol. Rev. 67, 1185–1248 (1987)
Renström, E., Eliasson, L. & Rorsman, P. Protein kinase A-dependent and -independent stimulation of exocytosis by cAMP in mouse pancreatic B-cells. J. Physiol. (Lond.) 502, 105–118 (1997)
Ozaki, N. et al. cAMP-GEFII is a direct target of cAMP in regulated exocytosis. Nature Cell Biol. 2, 805–811 (2000)
Gromada, J., Brock, B., Schmitz, O. & Rorsman, P. Glucagon-like peptide-1: regulation of insulin secretion and therapeutic potential. Basic Clin. Pharmacol. Toxicol. 95, 252–262 (2004)
Holz, G., Kühtreiber, W. & Habener, J. Pancreatic beta-cells are rendered glucose-competent by the insulinotropic hormone glucagon-like peptide-1(7-37). Nature 361, 362–365 (1993)
Eliasson, L. et al. SUR1 regulates PKA-independent cAMP-induced granule priming in mouse pancreatic B-cells. J. Gen. Physiol. 121, 181–197 (2003)
Gilon, P., Shepherd, R. & Henquin, J. C. Oscillations of secretion driven by oscillations of cytoplasmic Ca2+ as evidences in single pancreatic islets. J. Biol. Chem. 268, 22265–22268 (1993)
Bergsten, P., Grapengiesser, E., Gylfe, E., Tengholm, A. & Hellman, B. Synchronous oscillations of cytoplasmic Ca2+ and insulin release in glucose-stimulated pancreatic islets. J. Biol. Chem. 269, 8749–8753 (1994)
Steyer, J. A. & Almers, W. A real-time view of life within 100 nm of the plasma membrane. Nature Rev. Mol. Cell Biol. 2, 268–275 (2001)
Tengholm, A., Teruel, M. N. & Meyer, T. Single cell imaging of PI3K activity and glucose transporter insertion into the plasma membrane by dual colour evanescent wave microscopy. Sci. STKE PL4 (2003)
List, J. F. & Habener, J. Glucagon-like peptide 1 agonists and the development and growth of pancreatic β-cells. Am. J. Physiol. Endocrinol. Metab. 286, E875–E881 (2004)
Delmeire, D. et al. Type VIII adenylyl cyclase in rat beta cells: coincidence signal detector/generator for glucose and GLP-1. Diabetologia 46, 1383–1393 (2003)
Widmann, C., Bürki, E., Dolci, W. & Thorens, B. Signal transduction by the cloned glucagon-like peptide-1 receptor: comparison with signalling by the endogenous receptors of β cell lines. Mol. Pharmacol. 45, 1029–1035 (1994)
Ma, X. et al. Glucagon stimulates exocytosis in mouse and rat pancreatic α-cells by binding to glucagon receptors. Mol. Endocrinol. 19, 198–212 (2005)
Dyachok, O. & Gylfe, E. Ca2+-induced Ca2+ release via inositol 1,4,5-trisphosphate receptors is amplified by protein kinase A and triggers exocytosis in pancreatic β-cells. J. Biol. Chem. 279, 45455–45461 (2004)
Pyne, N. J. & Furman, B. L. Cyclic nucleotide phosphodiesterases in pancreatic islets. Diabetologia 46, 1179–1189 (2003)
Cooper, D. Regulation and organization of adenylyl cyclases and cAMP. Biochem. J. 375, 517–529 (2003)
Mehats, C., Andersen, C. B., Filopanti, M., Jin, S.-L. C. & Conti, M. Cyclic nucleotide phosphodiesterases and their role in endocrine cell signalling. Trends Endocrinol. Metab. 13, 29–35 (2002)
Cooper, D. M. F., Mons, N. & Karpen, J. W. Adenylyl cyclases and the interaction between calcium and cAMP signalling. Nature 374, 421–424 (1995)
Rich, T. C. & Karpen, J. W. Review article: cyclic AMP sensors in living cells: what signals can they actually measure? Ann. Biomed. Eng. 30, 1088–1099 (2002)
Pørksen, N. et al. Glucagon-like peptide increases mass but not frequency or orderliness of pulsatile insulin secretion. Diabetes 47, 45–49 (1998)
Ritzel, R. et al. Glucagon-like peptide 1 increases secretory burst mass of pulsatile insulin secretion in patients with type 2 diabetes and impaired glucose tolerance. Diabetes 50, 776–784 (2001)
Maeda, M. et al. Periodic signalling controlled by an oscillatory circuit that includes protein kinases ERK2 and PKA. Science 304, 875–878 (2004)
Gorbunova, Y. V. & Spitzer, N. C. Dynamic interactions of cyclic AMP transients and spontaneous Ca2+ spikes. Nature 418, 93–96 (2002)
Dolmetsch, R. E., Xu, K. & Lewis, R. S. Calcium oscillations increase the efficiency and specificity of gene expression. Nature 392, 933–936 (1998)
Harootunian, A. T. et al. Movement of the free catalytic subunit of cAMP-dependent protein kinase into and out of the nucleus can be explained by diffusion. Mol. Biol. Cell 4, 993–1002 (1993)
Griffin, B. A., Adams, S. R. & Tsien, R. Y. Specific covalent labeling of recombinant protein molecules inside living cells. Science 281, 269–272 (1998)
Asfari, M. et al. Establishment of 2-mercaptoethanol-dependent differentiated insulin-secreting cell lines. Endocrinology 130, 167–178 (1992)
Merglen, A. et al. Glucose-sensitivity and metabolism-secretion coupling studied during two-year continuous culture of INS-1E insulinoma cells. Endocrinology 145, 667–678 (2004)
Acknowledgements
We thank S. McKnight for providing cDNA for the regulatory and catalytic subunits of PKA; C. Wollheim and P. Maechler for the rat insulinoma cells; P. Korzhavyi for assistance with cross-correlation analysis; and E. Gylfe, C.-H. Heldin, B. Hellman and T. Meyer for reading the manuscript. This study was supported by grants from Åke Wiberg's Foundation, Carl Trygger's Foundation for Scientific Research, the European Foundation for the Study of Diabetes/Novo Nordisk, the Family Ernfors Foundation, Novo Nordisk Foundation, Swedish Diabetes Association, Swedish Research Council, and the Wenner–Gren Foundations.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Reprints and permissions information is available at npg.nature.com/reprintsandpermissions. The authors declare no competing financial interests.
Supplementary information
Supplementary Figures.
This file contains Supplementary Figures 1–6. Supplementary Figures 1 and 2 describe the construction, specificity and kinetic properties of the cAMP translocation biosensor. Supplementary Figures 3 and 4 show effects of norepinephrine, GLP-1 and glucagon on [cAMP]i. Supplementary Figure 5 shows a cross-correlation analysis of [cAMP]i and [Ca2+]i oscillations and Supplementary Figure 6 describes the contruction and FlAsH-labelling of tetracysteine-tagged PKA Cα. (PDF 698 kb)
Supplementary Methods.
This file contains a detailed description of the fluorescence microscopy techniques used in this study. (DOC 20 kb)
Rights and permissions
About this article
Cite this article
Dyachok, O., Isakov, Y., Sågetorp, J. et al. Oscillations of cyclic AMP in hormone-stimulated insulin-secreting β-cells. Nature 439, 349–352 (2006). https://doi.org/10.1038/nature04410
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature04410
This article is cited by
-
Subcellular second messenger networks drive distinct repellent-induced axon behaviors
Nature Communications (2023)
-
Glucose controls glucagon secretion by directly modulating cAMP in alpha cells
Diabetologia (2019)
-
Optical approaches for single-cell and subcellular analysis of GPCR–G protein signaling
Analytical and Bioanalytical Chemistry (2019)
-
Optical tools for understanding the complexity of β-cell signalling and insulin release
Nature Reviews Endocrinology (2018)
-
Red fluorescent protein-based cAMP indicator applicable to optogenetics and in vivo imaging
Scientific Reports (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.