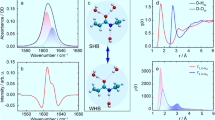
Abstract
Much progress has been made in our understanding of water molecule reactions on surfaces1, proton solvation in gas-phase water clusters2,3 and proton transfer through liquids4. Compared with our advanced understanding of these physico-chemical systems, much less is known about individual water molecules and their cooperative behaviour in heterogeneous proteins during enzymatic reactions. Here we use time-resolved Fourier transform infrared5 spectroscopy (trFTIR) and in situ H218O/H216O exchange FTIR to determine how the membrane protein bacteriorhodopsin6 uses the interplay among strongly hydrogen-bonded water molecules, a water molecule with a dangling hydroxyl group and a protonated water cluster7 to transfer protons. The precise arrangement of water molecules in the protein matrix results in a controlled Grotthuss proton transfer, in contrast to the random proton migration that occurs in liquid water. Our findings support the emerging paradigm that intraprotein water molecules are as essential for biological functions as amino acids.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Marx, D. Throwing tetrahedral dice. Science 303, 634–636 (2004)
Zwier, T. S. The structure of protonated water clusters. Science 304, 1119–1120 (2004)
Headrick, J. M. et al. Spectral signatures of hydrated proton vibrations in water clusters. Science 308, 1765–1769 (2005)
Marx, D., Tuckerman, M. E., Hutter, J. & Parrinello, M. The nature of the hydrated excess proton in water. Nature 397, 601–604 (1999)
Kötting, C. & Gerwert, K. Proteins in action monitored by time-resolved FTIR spectroscopy. Chem. Phys. Chem. 6, 881–888 (2005)
Lanyi, J. K. Bacteriorhodopsin. Annu. Rev. Physiol. 66, 665–688 (2004)
Garczarek, F., Brown, L. S., Lanyi, J. K. & Gerwert, K. Proton binding within a membrane protein by a protonated water cluster. Proc. Natl Acad. Sci. USA 102, 3633–3638 (2005)
de Grotthuss, C. J. T. Sur la décomposition de l'eau et des corps quélletient en dissolution à l'aide de l'électricité galvanique. Ann. Chim. 58, 54–74 (1806)
Eigen, M. Proton transfer, acid-base catalysis, and enzymatic hydrolysis. Part I: Elementary processes. Angew. Chem. Int. Edn Engl. 3, 1–19 (1964)
Zundel, G. in The Hydrogen Bond—Recent Developments in Theory and Experiments (ed. Sandorfy, C.) 683–766 (Nort-Holland, Amsterdam, 1976)
Birge, R. R. et al. Revised assignment of energy storage in the primary photochemical event in bacteriorhodopsin. J. Am. Chem. Soc. 113, 4327–4328 (1991)
Gerwert, K., Hess, B., Soppa, J. & Oesterhelt, D. Role of aspartate-96 in proton translocation by bacteriorhodopsin. Proc. Natl Acad. Sci. USA 86, 4943–4947 (1989)
Luecke, H., Schobert, B., Richter, H. T., Cartailler, J. P. & Lanyi, J. K. Structure of bacteriorhodopsin at 1.55 Å resolution. J. Mol. Biol. 291, 899–911 (1999)
Kandt, C., Schlitter, J. & Gerwert, K. Dynamics of water molecules in the bacteriorhodopsin trimer in explicit lipid/water environment. Biophys. J. 86, 705–717 (2004)
Rammelsberg, R., Huhn, G., Lubben, M. & Gerwert, K. Bacteriorhodopsins intramolecular proton-release pathway consists of a hydrogen-bonded network. Biochemistry 37, 5001–5009 (1998)
Dioumaev, A. K. et al. Existence of a proton transfer chain in bacteriorhodopsin: participation of Glu-194 in the release of protons to the extracellular surface. Biochemistry 37, 2496–2506 (1998)
Shibata, M. & Kandori, H. FTIR studies of internal water molecules in the Schiff base region of bacteriorhodopsin. Biochemistry 44, 7406–7413 (2005)
Hayashi, S. & Ohmine, I. Proton transfer in bacteriorhodopsin: Structure, excitation, IR spectra, and potential energy surface analyses by an ab initio QM/MM method. J. Phys. Chem. B 104, 10678–10691 (2000)
Liu, K., Brown, M. G., Cruzan, J. D. & Saykally, R. J. Vibration-rotation tunneling spectra of the water pentamer: Structure and dynamics. Science 271, 62–64 (1996)
Dencher, N. A., Sass, H. J., Buldt, G. Water and bacteriorhodopsin: structure, dynamics, and function. Biochim. Biophys. Acta 1460, 192–203 (2000)
Grudinin, S., Buldt, G., Gordeliy, V. & Baumgaertner, A. Water molecules and hydrogen-bonded networks in bacteriorhodopsin—molecular dynamics simulations of the ground state and the M intermediate. Biophys. J. 88, 3252–3261 (2005)
Le Coutre, J., Tittor, J., Oesterhelt, D. & Gerwert, K. Experimental evidence for hydrogen-bonded network proton transfer in bacteriorhodopsin shown by Fourier-transform infrared spectroscopy using azide as catalyst. Proc. Natl Acad. Sci. USA 92, 4962–4966 (1995)
Garczarek, F., Wang, J., El-Sayed, M. A. & Gerwert, K. The assignment of the different infrared continuum absorbance changes observed in the 3000–1800 cm-1 region during the bacteriorhodopsin photocycle. Biophys. J. 87, 2676–2682 (2004)
Hayashi, S., Tajkhorshid, E., Kandori, H. & Schulten, K. Role of hydrogen-bond network in energy storage of bacteriorhodopsin's light-driven proton pump revealed by ab initio normal-mode analysis. J. Am. Chem. Soc. 126, 10516–10517 (2004)
Tanimoto, T., Furutani, Y. & Kandori, H. Structural changes of water in the Schiff base region of bacteriorhodopsin: proposal of a hydration switch models. Biochemistry 42, 2300–2306 (2003)
Rozenberg, M., Loewenschuss, A. & Marcus, Y. An empirical correlation between stretching vibration redshift and hydrogen bond length. Phys. Chem. Chem. Phys. 2, 2699–2702 (2000)
Rousseau, R., Kleinschmidt, V., Schmitt, U. W. & Marx, D. Unravelling water network protonation patterns in bacteriorhodopsin by theoretical IR spectroscopy. Angew. Chem. Int. Edn Engl. 43, 4804–4807 (2004)
Spassov, V. Z., Luecke, H., Gerwert, K. & Bashford, D. pKa calculations suggest storage of an excess proton in a hydrogen-bonded water network in bacteriorhodopsin. J. Mol. Biol. 312, 203–219 (2001)
Luecke, H., Schobert, B., Richter, H. T., Cartailler, J. P. & Lanyi, J. K. Structural changes in bacteriorhodopsin during ion transport at 2 Å resolution. Science 286, 255–260 (1999)
Koradi, R., Billeter, M. & Wüthrich, K. MOLMOL: a program for display and analysis of macromolecular structures. J. Mol. Graph. 14, 51–55 (1996)
Acknowledgements
We thank A. Hartz for protein preparation; E. Hofmann and C. Kandt for help with figure preparation; and R. S. Goody and D. Rumschitzki for help with English. This work was funded by the Deutsche Forschungsgemeinschaft.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Reprints and permissions information is available at npg.nature.com/reprintsandpermissions. The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Garczarek, F., Gerwert, K. Functional waters in intraprotein proton transfer monitored by FTIR difference spectroscopy. Nature 439, 109–112 (2006). https://doi.org/10.1038/nature04231
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature04231
This article is cited by
-
Chasing weakly-bound biological water in aqueous environment near the peptide backbone by ultrafast 2D infrared spectroscopy
Communications Chemistry (2024)
-
Transient water wires mediate selective proton transport in designed channel proteins
Nature Chemistry (2023)
-
Protonation of Asp116 and distortion of the all-trans retinal chromophore in Krokinobacter eikastus rhodopsin 2 causes a redshift in absorption maximum upon dehydration
Photochemical & Photobiological Sciences (2023)
-
My remembrances of H.G. Khorana: exploring the mechanism of bacteriorhodopsin with site-directed mutagenesis and FTIR difference spectroscopy
Biophysical Reviews (2023)
-
Interdisciplinary biophysical studies of membrane proteins bacteriorhodopsin and rhodopsin
Biophysical Reviews (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.