Abstract
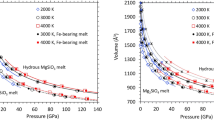
The chemical evolution of the Earth and the terrestrial planets is largely controlled by the density of silicate melts. If melt density is higher than that of the surrounding solid, incompatible elements dissolved in the melt will be sequestered in the deep mantle1,2. Previous studies on dry (water-free) melts showed that the density of silicate melts can be higher than that of surrounding solids under deep mantle conditions3,4,5,6,7,8. However, melts formed under deep mantle conditions are also likely to contain some water2, which will reduce the melt density. Here we present data constraining the density of hydrous silicate melt at the conditions of ∼410 km depth. We show that the water in the silicate melt is more compressible than the other components, and therefore the effect of water in reducing melt density is markedly diminished under high-pressure conditions. Our study indicates that there is a range of conditions under which a (hydrous) melt could be trapped at the 410-km boundary and hence incompatible elements could be sequestered in the deep mantle, although these conditions are sensitive to melt composition as well as the composition of the surrounding mantle.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Stolper, E. M., Walker, D., Hager, B. H. & Hays, J. F. Melt segregation from partially molten source regions: the importance of melt density and source region size. J. Geophys. Res. 86, 6261–6271 (1981)
Bercovici, D. & Karato, S. Whole-mantle convection and the transition-zone water filter. Nature 425, 39–44 (2003)
Agee, C. B. & Walker, D. Olivine flotation in mantle melt. Phys. Earth Planet. Inter. 114, 315–324 (1988)
Ohtani, E., Suzuki, A. & Kato, T. Flotation of olivine in the peridotite melt at high pressure. Proc. Jpn Acad. 69, 23–28 (1993)
Suzuki, A., Ohtani, E. & Kato, T. Flotation of diamond in mantle melt at high pressure. Science 269, 216–218 (1995)
Suzuki, A., Ohtani, E. & Kato, T. Density and thermal expansion of a peridotite melt at high pressure. Phys. Earth Planet. Inter. 107, 53–61 (1998)
Suzuki, A. & Ohtani, E. Density of peridotite melts at high pressure. Phys. Chem. Miner. 30, 449–456 (2003)
Ohtani, E. & Maeda, M. Density of basaltic melt at high pressure and stability of the melt at the base of the lower mantle. Phys. Earth Planet. Inter. 193, 69–75 (2001)
Ochs, F. A. III & Lange, R. A. The partial molar volume, thermal expansivity, and compressibility of H2O in NaAlSi3O8 liquid: new measurements and an internally consistent model. Contrib. Mineral. Petrol. 129, 155–165 (1997)
Inoue, T. & Sawamoto, H. in High-pressure Research: Application to Earth and Planetary Sciences (eds Shono, Y. & Manghnani, H.) 323–331 (Terra Scientific, Tokyo/American Geophysical Union, Washington DC, 1992)
Inoue, T. Effect of water on melting phase relations and melt composition in the system Mg2SiO4-MgSiO3-H2O up to 15 GPa. Phys. Earth Planet. Inter. 85, 237–263 (1994)
Litasov, K. & Ohtani, E. Phase relations and melt compositions in CMAS-pyrolite-H2O system up to 25 GPa. Phys. Earth Planet. Inter. 134, 105–127 (2002)
Takahashi, E., Shimazaki, T., Tsuzaki, Y. & Yoshida, H. Melting study of a peridotite KLB-1 to 6.5 GPa and the origin of basaltic magmas. Phil. Trans. R. Soc. Lond. A 342, 105–120 (1993)
Matsukage, K. N. & Kubo, K. Chromian spinel during melting experiments of dry peridotite (KLB-1) at 1.0-2.5 GPa. Am. Mineral. 88, 1271–1278 (2003)
Bottinga, Y. & Weill, D. F. Densities of liquid silicate systems calculated from partial molar volumes of oxide components. Am. J. Sci. 269, 169–182 (1970)
Burnham, C. W. & Davis, N. F. The role of H2O in silicate melts: I P-V-T relations in the system NaAlSi3O8-H2O to 10 kilobars and 1000°C. Am. J. Sci. 270, 54–79 (1971)
Ito, E. & Katsura, T. A temperature profile of the mantle transition zone. Geophys. Res. Lett. 16, 425–528 (1989)
Dziewonski, A. M. & Anderson, D. L. Preliminary reference Earth model. Phys. Earth Planet. Inter. 25, 297–356 (1981)
Matsukage, K. N., Nishihara, Y. & Karato, S. Seismological signature of chemical differentiation of Earth's upper mantle. J. Geophys. Res. (in the press)
Nishihara, Y. & Takahashi, E. Phase relation and physical properties of an Al-depleted komatiite to 23 GPa. Earth Planet. Sci. Lett. 190, 65–77 (2001)
Fei, Y. in Mineral Physics & Crystallography, A Handbook of Physical Constants (ed. Ahrens, T. J.) 29–44 (American Geophysical Union, Washington DC, 1995)
Agee, C. Crystal-liquid density inversions in terrestrial and lunar magmas. Phys. Earth Planet. Inter. 107, 63–74 (1998)
Nishihara, Y., Shinmei, T. & Karato, S. Grain-growth kinetics in wadsleyite: effects of chemical environment. Phys. Earth Planet. Inter. (in the press)
Zhang, J., Li, B., Utsumi, W. & Liberman, R. C. In situ X-ray observations of the coesite-stishovite transition: reversed phase boundary and kinetics. Phys. Chem. Miner. 23, 1–10 (1996)
Morishima, H. et al. The phase boundary between α-Mg2SiO4 and β-Mg2SiO4 determined by in-situ X-ray observation. Science 265, 1202–1203 (2004)
Li, J., Hadidiacos, C., Mao, H-K., Fei, Y. & Hemley, R. J. Effect of pressure on thermocouples in a multi-anvil apparatus. High Press. Res. 23, 389–401 (2003)
Lange, R. A. & Carmichael, I. S. E. Density of Na2O-K2O-CaO-MgO-FeO-Fe2O3-Al2O3-TiO2-SiO2 liquids: new measurements and derived partial molar properties. Geochim. Cosmochim. Acta 51, 2931–2946 (1987)
Lange, R. A. A revised model for the density and thermal expansivity of K2O-Na2O-CaO-MgO-Al2O3-SiO2 liquids from 700 to 1900 K: extension to crustal magmatic temperatures. Contrib. Mineral. Petrol. 130, 1–11 (1997)
Ochs, F. A. III & Lange, R. A. The density of hydrous magmatic liquid. Science 283, 1314–1317 (1999)
Richet, P. et al. Water and the density of silicate glasses. Contrib. Mineral. Petrol. 138, 337–347 (2000)
Acknowledgements
We thank Y. Nishihara for advice on experimental techniques and discussions, and H. Sumiya for the supply of single crystals of diamond. This work was supported by NSF (S.K.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Reprints and permissions information is available at npg.nature.com/reprintsandpermissions. The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Matsukage, K., Jing, Z. & Karato, Si. Density of hydrous silicate melt at the conditions of Earth's deep upper mantle. Nature 438, 488–491 (2005). https://doi.org/10.1038/nature04241
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature04241
This article is cited by
-
Preparation of Al2O3–Cr2O3 Solid Solutions as Buoyancy Markers and Their High Pressure Synchrotron X-ray Diffraction Analysis
Pure and Applied Geophysics (2022)
-
Behavior and properties of water in silicate melts under deep mantle conditions
Scientific Reports (2021)
-
Theoretical models and experimental determination methods for equations of state of silicate melts: A review
Science China Earth Sciences (2019)
-
Deep and persistent melt layer in the Archaean mantle
Nature Geoscience (2018)
-
Water makes glass elastically stiffer under high-pressure
Scientific Reports (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.