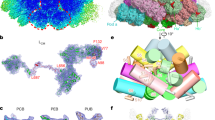
Abstract
Phytochromes are red/far-red light photoreceptors that direct photosensory responses across the bacterial, fungal and plant kingdoms. These include photosynthetic potential and pigmentation in bacteria as well as chloroplast development and photomorphogenesis in plants. Phytochromes consist of an amino-terminal region that covalently binds a single bilin chromophore, followed by a carboxy-terminal dimerization domain that often transmits the light signal through a histidine kinase relay. Here we describe the three-dimensional structure of the chromophore-binding domain of Deinococcus radiodurans phytochrome assembled with its chromophore biliverdin in the Pr ground state. Our model, refined to 2.5 Å resolution, reaffirms Cys 24 as the chromophore attachment site, locates key amino acids that form a solvent-shielded bilin-binding pocket, and reveals an unusually formed deep trefoil knot that stabilizes this region. The structure provides the first three-dimensional glimpse into the photochromic behaviour of these photoreceptors and helps to explain the evolution of higher plant phytochromes from prokaryotic precursors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Vierstra, R. D. & Karniol, B. in Handbook of Photosensory Receptors (eds Briggs, W. R. & Spudich, J. L.) 171–196 (Wiley, Weinheim, 2005)
Quail, P. H. Phytochrome photosensory signalling networks. Nature Rev. Mol. Cell Biol. 3, 85–93 (2002)
Smith, H. Physiological and ecological functions with the phytochrome family. Annu. Rev. Plant Physiol. Plant Mol. Biol. 46, 269–315 (1995)
Karniol, B., Wagner, J. R., Walker, J. M. & Vierstra, R. D. Phylogenetic analysis of the phytochrome superfamily reveals distinct microbial subfamilies of photoreceptors. Biochem. J. 392, 103–116 (2005)
Davis, S. J., Vener, A. V. & Vierstra, R. D. Bacteriophytochromes: phytochrome-like photoreceptors from nonphotosynthetic eubacteria. Science 286, 2517–2520 (1999)
Wu, S. H. & Lagarias, J. C. Defining the bilin lyase domain: lessons from the extended phytochrome superfamily. Biochemistry 39, 13487–13495 (2000)
Cherry, J. R. et al. Carboxy-terminal deletion analysis of oat phytochrome A reveals the presence of separate domains required for structure and biological activity. Plant Cell 5, 565–575 (1993)
Bateman, A. et al. The Pfam protein families database. Nucleic Acids Res. 32D, 138–141 (2004)
Yildiz, O. et al. Crystal structure and interactions of the Pas repeat region of the Drosophila clock protein Period. Mol. Cell 17, 69–82 (2005)
Razeto, A. et al. Structure of the Ncoa-1/Src-1 Pas-B domain bound to the Lxxll motif of the Stat6 transactivation domain. J. Mol. Biol. 336, 319–329 (2004)
Lamparter, T. et al. The biliverdin chromophore binds covalently to a conserved cysteine residue in the N-terminus of Agrobacterium phytochrome Agp1. Biochemistry 43, 3659–3669 (2004)
Bhoo, S. H. et al. Phytochrome photochromism probed by site-directed mutations and chromophore esterification. J. Am. Chem. Soc. 48, 11717–11718 (1997)
Taylor, W. R. A deeply knotted protein structure and how it might fold. Nature 406, 916–919 (2000)
Nureki, O. et al. An enzyme with a deep trefoil knot for the active-site architecture. Acta Crystallogr. D 58, 1129–1137 (2002)
Taylor, W. R. & Lin, K. Protein knots: a tangled problem. Nature 421, 25 (2003)
Zarembinski, T. I. et al. Deep trefoil knot implicated in RNA binding found in an archaebacterial protein. Proteins Struct. Funct. Genet. 50, 177–183 (2003)
Mallam, A. L. & Jackson, S. E. Folding studies on a knotted protein. J. Mol. Biol. 346, 1409–1421 (2005)
Liu, Y. & Eisenberg, D. 3D domain swapping: as domains continue to swap. Protein Sci. 11, 1285–1299 (2002)
Vaguine, A. A., Richelle, J. & Wodak, S. J. SFCHECK: a unified set of procedures for evaluating the quality of macromolecular structure-factor data and their agreement with the atomic model. Acta Crystallogr. D 55, 191–205 (1999)
Kneip, C. et al. Protonation state and structural changes of the tetrapyrrole chromophore during the Pr → Pfr phototransformation of phytochrome: a resonance Raman spectroscopic study. Biochemistry 38, 15185–15192 (1999)
Mroginski, M. A. et al. Determination of the chromophore structures in the photoinduced reaction cycle of phytochrome. J. Am. Chem. Soc. 126, 16734–16735 (2004)
Braslavsky, S. E. in Photochromisms, Molecules and Systems (eds BrouasLaurent, H. & BrouasLaurent, D. H.) 738–755 (Elsevier Science, Amsterdam, 2003)
Duerring, M., Schmidt, G. B. & Huber, R. Isolation, crystallization, crystal structure analysis and refinement of constitutive C-phycocyanin from the chromatically adapting cyanobacterium Fremyella diplosiphon at 1.66 Å resolution. J. Mol. Biol. 217, 577–592 (1991)
Brejc, K., Ficner, R., Huber, R. & Steinbacher, S. Isolation, crystallization, crystal structure analysis and refinement of allophycocyanin from the cyanobacterium Spirulina platensis at 2.3 Å resolution. J. Mol. Biol. 249, 424–440 (1995)
Hanzawa, H. et al. In vitro assembly of phytochrome B apoprotein with synthetic analogs of the phytochrome chromophore. Proc. Natl Acad. Sci. USA 98, 3612–3617 (2001)
Tu, S.-L. & Lagarias, J. C. in Handbook of Photosensory Receptors (eds Briggs, W. R. & Spudich, J. L.) 121–149 (Wiley, Weinheim, 2005)
Fodor, S. P., Lagarias, J. C. & Mathies, R. A. Resonance Raman analysis of the Pr and Pfr forms of phytochrome. Biochemistry 29, 11141–11146 (1990)
Inomata, K. et al. Sterically locked synthetic bilin derivatives and phytochrome Agp1 from Agrobacterium tumefaciens form photosensitive Pr- and Pfr-like adducts. J. Biol. Chem. 280, 24491–24497 (2005)
Gartner, W. & Braslavsky, S. E. in Photoreceptors in Light Signaling (ed. Baschauer, A.) 136–180 (Royal Soc. Chemistry, Cambridge, UK, 2004)
Cheng, M., Tao, Y., Lim, J., Shaw, A. & Chory, J. Regulation of phytochrome B nuclear localization through light-dependent unmasking of nuclear-localization signals. Curr. Biol. 15, 637–642 (2005)
Ryu, J. S. et al. Phytochrome-specific type 5 phosphatase controls light signal flux by enhancing phytochrome stability and affinity for a signal transducer. Cell 120, 395–406 (2005)
Lamparter, T. et al. Biliverdin binds covalently to Agrobacterium phytochrome Agp1 via its ring A vinyl side chain. J. Biol. Chem. 278, 33786–33792 (2003)
Fischer, A. J. & Lagarias, J. C. Harnessing phytochrome's glowing potential. Proc. Natl Acad. Sci. USA 101, 17334–17339 (2004)
Mutsuda, M., Michel, K. P., Zhang, X. F., Montgomery, B. L. & Golden, S. S. Biochemical properties of CikA, an unusual phytochrome-like histidine protein kinase that resets the circadian clock in Synechococcus elongatus PCC 7942. J. Biol. Chem. 278, 19102–19110 (2003)
Terauchi, K., Montgomery, B. L., Grossman, A. R., Lagarias, J. C. & Kehoe, D. M. RcaE is a complementary chromatic adaptation photoreceptor required for green and red light responsiveness. Mol. Microbiol. 51, 567–577 (2004)
Yoshihara, S., Katayama, M., Geng, X. X. & Ikeuchi, M. Cyanobacterial phytochrome-like PixJ1 holoprotein shows novel reversible photoconversion between blue- and green-absorbing forms. Plant Cell Physiol. 45, 1729–1737 (2004)
Lagarias, J. C. & Rapoport, H. Chromopeptides from phytochrome. The structure and linkage of the Pr form of the phytochrome chromophore. J. Am. Chem. Soc. 102, 4821–4828 (1980)
Yeh, K. C., Wu, S. H., Murphy, J. T. & Lagarias, J. C. A cyanobacterial phytochrome two-component light sensory system. Science 277, 1505–1508 (1997)
Bhoo, S. H., Davis, S. J., Walker, J., Karniol, B. & Vierstra, R. D. Bacteriophytochromes are photochromic histidine kinases using a biliverdin chromophore. Nature 414, 776–779 (2001)
Ramakrishnan, V., Finch, J. T., Graziano, V., Lee, P. L. & Sweet, R. M. Crystal structure of globular domain of histone H5 and its implications for nucleosome binding. Nature 362, 219–223 (1993)
Chayen, N. E., Shaw-Stewart, P. D. & Blow, D. M. Microbatch crystallization under oil—a new technique allowing many small-volume crystallization trials. J. Cryst. Growth 122, 176–180 (1992)
Otwinowski, Z. & Minor, W. in Macromolecular Crystallography Part A (eds Carter, C. W. Jr & Sweet, R. M.) 307–326 (Academic, New York, 1997)
Grosse-Kunstleve, R. W. & Adams, P. D. Substructure search procedures for macromolecular structures. Acta Crystallogr. D 59, 1974–1977 (2003)
Bricogne, G., Vonrhein, C., Flensburg, C., Schiltz, M. & Paciorek, W. Generation, representation and flow of phase information in structure determination: recent developments in and around SHARP 2.0. Acta Crystallogr. D 59, 2023–2030 (2003)
Perrakis, A., Morris, R. M. & Lamzin, V. S. Automated protein model building combined with iterative structure refinement. Nature Struct. Biol. 6, 458–463 (1999)
Terwilliger, T. SOLVE and RESOLVE: automated structure solution, density modification, and model building. J. Synch. Radiat. 11, 49–52 (2004)
McRee, D. E. XtalView Xfit—A versatile program for manipulating atomic coordinates and electron density. J. Struct. Biol. 125, 156–165 (1999)
Winn, M. D., Isupov, M. N. & Murshudov, G. N. Use of TLS parameters to model anisotropic displacements in macromolecular refinement. Acta Crystallogr. D 57, 122–133 (2001)
Acknowledgements
We thank B. Karniol, S. Beale and K. Satyshur for technical advice and acknowledge the Michigan Economic Development Corporation and the Michigan Technology Tri-Corridor for the support of this research program. Use of the Advanced Photon Source was supported by the US Department of Energy, Office of Science, Office of Basic Energy Sciences. This work was supported by grants from the US National Science Foundation (R.D.V. and K.T.F.), the US Department of Energy (R.D.V.), and the W.M. Keck Foundation (K.T.F.). Author Contributions J.R.W. purified protein and grew and characterized crystals; J.S.B. collected data and carried out initial phase determination; K.T.F. and J.R.W. phased, modelled, refined and validated structure; R.D.V. initiated collaboration; K.T.F. designed structure experiments; J.R.W., K.T.F. and R.D.V. interpreted the structure and prepared the manuscript and figures.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Atomic coordinates and structure factor amplitudes have been deposited in the Protein Data Bank (accession code 1ZTU). Reprints and permissions information is available at npg.nature.com/reprintsandpermissions. The authors declare no competing financial interests.
Supplementary information
Supplementary Table 1
This table includes all data collection and scaling statistics as well as refinement statistics for the crystal structure determination of phytochrome. (DOC 48 kb)
Supplementary Figure 1
Spectra of native, selenomethionine labeled, and crystalline DrCBD. (PDF 266 kb)
Supplementary Figure 2
Sequence alignment of plant, cyanobacterial and bacterial phytochromes with secondary structure elements indicated (PDF 300 kb)
Rights and permissions
About this article
Cite this article
Wagner, J., Brunzelle, J., Forest, K. et al. A light-sensing knot revealed by the structure of the chromophore-binding domain of phytochrome. Nature 438, 325–331 (2005). https://doi.org/10.1038/nature04118
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature04118
This article is cited by
-
Evidence for an early green/red photocycle that precedes the diversification of GAF domain photoreceptor cyanobacteriochromes
Photochemical & Photobiological Sciences (2023)
-
Responding to light signals: a comprehensive update on photomorphogenesis in cyanobacteria
Physiology and Molecular Biology of Plants (2023)
-
Protein control of photochemistry and transient intermediates in phytochromes
Nature Communications (2022)
-
Plant phytochrome B is an asymmetric dimer with unique signalling potential
Nature (2022)
-
Using fluorescent promoter-reporters to study sugar utilization control in Bifidobacterium longum NCC 2705
Scientific Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.