Abstract
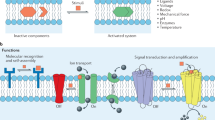
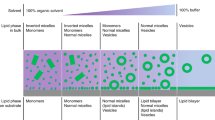
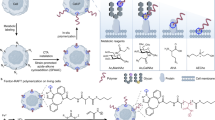
Lipid-bilayer membranes supported on solid substrates are widely used as cell-surface models that connect biological and artificial materials. They can be placed either directly on solids or on ultrathin polymer supports that mimic the generic role of the extracellular matrix. The tools of modern genetic engineering and bioorganic chemistry make it possible to couple many types of biomolecule to supported membranes. This results in sophisticated interfaces that can be used to control, organize and study the properties and function of membranes and membrane-associated proteins. Particularly exciting opportunities arise when these systems are coupled with advanced semiconductor technology.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Brian, A. A. & McConnell, H. M. Allogeneic stimulation of cytotoxic T cells by supported planar membranes. Proc. Natl Acad. Sci. USA 81, 6159–6163 ( 1984).
Chan, P. et al. Influence of receptor lateral mobility on adhesion strengthening between membranes containing LFA-3 and CD2. J. Cell Biol. 10, 245–255 ( 1991).
Erb, E. -M., Tangemann, K., Bohrmann, B., Müller, B. & Engel, J. Integrin 4IIbM3 reconstituted into lipid bilayers is nonclustered in its activated state but clusters after fibrinogen binding. Biochemistry 36, 7395–7402 ( 1997).
Kloboucek, A., Behrisch, A., Faix, J. & Sackmann, E. Adhesion-induced receptor segration and adhesion plaque formation: A model membrane study. Biophys. J. 77, 2311–2328 ( 1999).
Qi, S. Y., Groves, J. T. & Chakraborty, A. K. Synaptic pattern formation during cellular recognition. Proc. Natl Acad. Sci. USA 98, 6548–6553 ( 2001).
Grakoui, A. et al. The immunological synapse: A molecular machine controlling T cell activation. Science 285, 221–227 ( 1999).
, K. & McConnell, H. M. Supported phospholipid bilayers. Biophys. J. 47, 105–113 ( 1985).
Groves, J. T. & Dustin, M. L. Supported planar bilayers in studies on immune cell adhesion and communication. J. Immunol. Meth. 278, 19–32 ( 2003).
Sackmann, E. Supported membranes: Scientific and practical applications. Science 271, 43–48 ( 1996).
Groves, J. T. & Boxer, S. G. Micropattern formation in supported lipid membranes. Acc. Chem. Res. 35, 149–157 ( 2002).
Watts, T. H., Gaub, H. E. & McConnell, H. M. T-cell-mediated association of peptide antigen and major histocompatibility complex protein detected by energy-transfer in an evanescent wave-field. Nature 320, 179–181 ( 1986).
Kalb, E., Frey, S. & Tamm, L. K. Formation of supported planar bilayers by fusion of vesicles to supported phospholipid monolayers. Biochim. Biophys. Acta 1103, 307–316 ( 1992).
Bayerl, T. M. & Bloom, M. Physical-properties of single phospholipid-bilayers adsorbed to micro glass-beads — a new vesicular model system studied by H-2-nuclear magnetic-resonance. Biophys. J. 58, 357–362 ( 1990).
Tatulian, S. A., Hinterdorfer, P., Baber, G. & Tamm, L. K. Influenza hemagglutinin assumes a tilted conformation during membrane-fusion as determined by attenuated total-reflection FTIR spectroscopy. EMBO J. 14, 5514–5523 ( 1995).
Terrettaz, S., Stora, T., Duschl, C. & Vogel, H. Protein-binding to supported lipid-membranes — Investigation of the cholera-toxin ganglioside interaction by simultaneous impedance spectroscopy and surface-plasmon resonance. Langmuir 9, 1361–1369 ( 1993).
Kjaer, K., Als-Nielsen, J., Helm, C. A., Laxhuber, L. A. & Mohwald, H. Ordering in lipid monolayers studied by synchrotron X-ray-diffraction and fluorescence microscopy. Phys. Rev. Lett. 58, 2224–2227 ( 1987).
Johnson, S. J. et al. Structure of an adsorbed dimyristoylphosphatidylcholine bilayer measured with specular reflection of neutrons. Biophys. J. 59, 289–294 ( 1991).
Kalb, E., Engel, J. & Tamm, L. K. Binding of proteins to specific target sites in membranes measured by total internal-reflection fluorescence microscopy. Biochemistry 29, 1607–1613 ( 1990).
Bruinsma, R., Behrisch, A. & Sackmann, E. Adhesive switching of membranes: Experiment and theory. Phys. Rev. E 61, 4253–4267 ( 2000).
Sackmann, E. & Bruinsma, R. F. Cell adhesion as wetting transition? Chem. Phys. Chem. 3, 262–269 ( 2002).
Wagner, M. L. & Tamm, L. K. Reconstituted syntaxin1A/SNAP25 interacts with negatively charged lipids as measured by lateral diffusion in planar supported bilayers. Biophys. J. 61, 266–275 ( 2001).
Sackmann, E. & Tanaka, M. Supported membranes on soft polymer cushions: Fabrication, characterization and applications. Trends Biotechnol. 18, 58–64 ( 2000).
Knoll, W. et al. Functional tethered lipid bilayers. Rev. Mol. Biotechnol. 74, 137–158 ( 2000).
Koenig, B. W. et al. Neutron reflectivity and atomic force microscopy studies of a lipid bilayer in water adsorbed to the surface of a silicon single crystal. Langmuir 12, 1343–1350 ( 1996).
Lambacher, A. & Fromherz, P. Fluorescence interference-contrast microscopy on oxidized silicon using a monomolecular dye layer. Appl. Phys. A 63, 207–216 ( 1996).
Elender, G. & Sackmann, E. Wetting and dewetting of Si/SiO2-wafers by free and lipid-monolayer covered aqueous solutions under controlled humidity. J. Phys. II 4, 455–479 ( 1994).
Nissen, J., Gritsch, S., Wiegand, G. & Rädler, J. O. Wetting of phospholipid membranes on hydrophilic surfaces — concepts towards self-healing membranes. Eur. Phys. J. B 10, 335–344 ( 1999).
Tanaka, M. et al. Wetting and dewetting of extracellular matrix and glycocalix models. J. Phys. Cond. Matt. 17, S649S663 ( 2005).
Schaub, M., Wenz, G., Wegner, G., Stein, A. & Klemm, D. Ultrathin films of cellulose on silicon wafers. Adv. Mater. 5, 919–922 ( 1993).
Goennenwein, S., Tanaka, M., Hu, B., Moroder, L. & Sackmann, E. Functional incorporation of integrins into solid supported membranes on ultrathin films of cellulose: Impact on adhesion. Biophys. J. 85, 646–655 ( 2003).
Lang, H., Duschl, C. & Vogel, H. A new class of thiolipid for the attachment of lipid bilayers on gold surfaces. Langmuir 10, 197–210 ( 1994).
Cornell, B. A. et al. A biosensor that uses ion-channel switches. Nature 387, 580–583 ( 1997).
Schiller, S. M., Naumann, R., Lovejoy, K., Kunz, H. & Knoll, W. Archaea analogue thiolipids for tethered bilayer lipid membranes on ultrasmooth gold surfaces. Angew. Chem. Int. Ed. Engl. 42, 208–211 ( 2003).
Wagner, M. L. & Tamm, L. K. Tethered polymer-supported planar lipid bilayers for reconstitution of integral membrane proteins: Silane-polyethylenglycol-lipid as a cushion and covalent linker. Biophys. J. 79, 1400–1414 ( 2000).
Bunjes, N. et al. Thiopeptide-supported lipid layers on solid substrates. Langmuir 13, 6188–6194 ( 1997).
Purrucker, O., Förtig, A., Jordan, R. & Tanaka, M. Supported membranes with well-defined polymer tethers — Incorporation of cell receptors. Chem. Phys. Chem. 5, 327–335 ( 2004).
Purrucker, O., Förtig, A., Ludke, K., Jordan, R. & Tanaka, M. Confinement of transmembrane receptors in tunable stripe micropatterns. J. Am. Chem. Soc. 127, 1258–1264 ( 2005).
Fischer, M., Bacher, A., Haase, I., Tristl, M. & Sackmann, E. Design of biofunctional assemblies on solids through recombinant bacterial protein lumazine synthase. Chem. Phys. Chem. 2, 623–627 ( 2001).
Demè, B., Hess, D., Tristl, M., Lee, L. -T. & Sackmann, E. Binding of actin filaments to charged lipid monolayers: Film balance experiments combined with neutron reflectivity. Eur. Phys. J. E 2, 125–136 ( 2000).
Salafsky, J., Groves, J. T. & Boxer, S. G. Architecture and function of membrane proteins in planar supported bilayers: A study with photosyntehtic reaction centers. Biochemistry 35, 14773–14781 ( 1996).
Tanaka, M., Kaufmann, S., Nissen, J. & Hochrein, M. Orientation selective immobilization of human erythrocyte membranes on ultrathin cellulose films. Phys. Chem. Chem. Phys. 3, 4091–4095 ( 2001).
Tanaka, M., Wong, A. P., Rehfeldt, F., Tutus, M. & Kaufmann, S. Selective deposition of native cell membranes on biocompatible micro-patterns. J. Am. Chem. Soc. 126, 3257–3260 ( 2004).
Springer, T. A. Traffic signals on endothelium for lymphocyte recirculation and leukocyte emigration. Annu. Rev. Physiol. 57, 827–872 ( 1995).
Stelzle, M., Mielich, R. & Sackmann, E. Two-dimensional microelectrophoresis in supported lipid bilayers. Biophys. J. 63, 1346–1354 ( 1992).
Groves, J. T., Boxer, S. G. & McConnell, H. M. Electric field-induced reorganization of two-component supportedbilayer membranes. Proc. Natl Acad. Sci. USA 25, 13390–13395 ( 1997).
Groves, J. T., Wulfing, C. & Boxer, S. G. Electrical manipulation of glycan phosphatidyl inositol tethered protein in planar supported bilayers. Biophys. J. 71, 2716–2723 ( 1996).
Olson, D. J. et al. Elecrophoresis of DNA adsorbed to a cationic supported bilayer. Langmuir 17, 7396–7401 ( 2001).
Yoshina-Ishii, C. & Boxer, S. G. Arrays of mobile tethered vesicles on supported lipid bilayers. J. Am. Chem. Soc. 125, 3696–3697 ( 2003).
Merkel, R., Sackmann, E. & Evans, E. Molecular friction and epitactic coupling between monolayers in supported bilayers. J. Phys. (Paris) 50, 1535–1555 ( 1989).
Hillebrandt, H., Tanaka, M. & Sackmann, E. A novel membrane charge sensor: sensitive detection of surface charge at polymer/lipid composite films on indium-tin-oxide electrodes. J. Phys. Chem. B 106, 477–488 ( 2002).
McLaughlin, S. & Poo, M. M. The role of electro-osmosis in the electric-field-induced movement of charged macromolecules on the surfaces of cells. Biophys. J. 34, 85–93 ( 1981).
van Oudenaarden, A. & Boxer, S. G. Brownian ratchets: Molecular separations in lipid bilayers supported on patterned arrays. Science 285, 1046–1048 ( 1999).
Groves, J. T., Ulman, N. & Boxer, S. G. Micropatterning fluid lipid bilayers on solid supports. Science 275, 651–653 ( 1997).
Groves, J. T., Mahal, L. K. & Bertozzi, C. R. Control of cell adhesion and growth with micropatterned supported lipid membranes. Langmuir 17, 5129–5133 ( 2001).
Yang, T., Baryshnikova, O. K., Mao, H., Holden, M. A. & Cremer, P. S. Investigation of bivalent antibody binding on fluid-supported phospholipid bilayers: The effect of hapten density. J. Am. Chem. Soc. 125, 4779–4784 ( 2003).
Morigaki, K., Baumgart, T., Offenhausser, A. & Knoll, W. Patterning solid-supported lipid bilayer membranes by lithographic polymerization of a diacetylene lipid. Ang. Chem. Inter. Ed. 40, 172–174 ( 2001).
Hovis, J. S. & Boxer, S. G. Patterning barriers to lateral diffusion in supported lipid bilayer membranes by blotting and stamping. Langmuir 16, 894–897 ( 2000).
Sapuri, A. R., Baksh, M. M. & Groves, J. T. Electrostatically targeted intermembrane lipid exchange with micropatterned supported membranes. Langmuir 19, 1606–1610 ( 2003).
Kung, L. A., Kam, L., Hovis, J. S. & Boxer, S. G. Patterning hybrid surfaces of proteins and supported lipid bilayers. Langmuir 16, 6773–6776 ( 2000).
Rehfeldt, F. & Tanaka, M. Hydration forces in ultrathin films of cellulose. Langmuir 19, 1467–1473 ( 2003).
Dean, C. et al. Neurexin mediates the assembly of presynaptic terminals. Nature Neurosci. 6, 708–716 ( 2003).
Galneder, R. et al. Microelectrophoresis of a bilayer-coated silica bead in an optical trap: Application to enzymology. Biophys. J. 80, 2298–2309 ( 2001).
Loidl-Stahlhofen, A., Kaufmann, S., Braunschweig, T. & Bayerl, T. M. The thermodynamic control of protein binding to lipid bilayers for protein chromatography. Nature Biotechnol. 14, 999–1002 ( 1996).
Baksh, M. M., Jaros, M. & Groves, J. T. Detection of molecular interactions at membrane surfaces through colloid phase transitions. Nature 427, 139–141 ( 2004).
Jacobson, B. S. & Branton, D. Plasma membrane: rapid isolation and exposure of the cytoplasmic surface by use of positively charged beads. Science 195, 302–304 ( 1976).
Cohen, C. M., Kalish, D. I., Jacobson, B. S. & Branton, D. Membrane isolation on polylysine-coated beads. Plasma membrane from HeLa cells. J. Cell. Biol. 75, 119–134 ( 1977).
Kaufmann, S. & Tanaka, M. Cell adhesion onto highly curved surfaces: One-step immobilization of human erythrocyte membranes on silica beads. Chem. Phys. Chem. 4, 699–704 ( 2003).
Sakmann, B. & Neher, E., Single-channel Recording (Plenum, New York, 1985).
Fertig, N., Meyer, C., Blick, R. H., Trautmann, C. H. & Behrends, J. C. Microstructured glass chip for ion-channel electrophysiology. Phys. Rev. E 64, 040901 ( 2001).
Borisenko, V. et al. Simultaneous optical and electrical recording of single gramicidin channels. Biophys. J. 84, 612–622 ( 2003).
Plant, A. L., Gueguetchkeri, M. & Yap, W. Supported phospholipid/alkanethiol biomimetic membranes — Insulating properties. Biophys. J. 67, 1126–1133 ( 1994).
Steinem, C., Janshoff, A., Ulrich, W. -P., Sieber, M. & Galla, H. -J. Impedance analysis of supported lipid bilayer membranes: a scrutiny of different preparation techniques. Biochim. Biophys. Acta 1279, 169–180 ( 1996).
Stenberg, M., Arwin, H. & Nilsson, A. Silicon-silicon dioxide as an electrode for electrical and ellipsometric measurements of adsorbed organic molecules. J. Colloid Interface Sci. 72, 255–264 ( 1979).
Hillebrandt, H., Wiegand, G., Tanaka, M. & Sackmann, E. High electric resistance polymer/lipid composite films on indium-tin-oxide electrodes. Langmuir 15, 8451–8459 ( 1999).
Gritsch, S., Nollert, P., Jähnig, F. & Sackmann, E. Impedance spectroscopy of porin and gramicidin pores reconstituted into supported lipid bilayers on indium-tin-oxide electrodes. Langmuir 14, 3118–3125 ( 1998).
Purrucker, O., Hillebrandt, H., Adlkofer, K. & Tanaka, M. Deposition of highly resistive lipid bilayer on silicon — silicon dioxide electrode and incorporation of gramicidin studied by ac impedance spectroscopy. Electrochim. Acta 47, 791 ( 2001).
Wiegand, G., Arribas-Layton, N., Hillebrandt, H., Sackmann, E. & Wagner, P. Electrical properties of supported lipid bilayer membranes. J. Phys. Chem. B 106, 4245–4254 ( 2002).
Wiegand, W., Neumaier, K. R. & Sackmann, E. Fast impedance spectroscopy: General aspects and performance study for single ion channel measurements. Rev. Sci. Instrum. 71, 2309–2320 ( 2000).
Fromherz, P., Offenhausser, A., Vetter, T. & Weis, J. A neuron-silicon junction — a Retzius cell of the leech on an insulated-gate field-effect transistor. Science 252, 1290–1293 ( 1991).
Straub, B., Meyer, E. & Fromherz, P. Recombinant maxi-K channels on transistor, a prototype of iono-electronic interfacing. Nature Biotechnol. 19, 121–124 ( 2001).
Steinhoff, G., Purrucker, O., Tanaka, M., Stutzmann, M. & Eickhoff, M. AlxGa1-xN — A new material system for biosensors. Adv. Funct. Mater. 13, 841–846 ( 2003).
Steinhoff, G. et al. Recording of cell action potentials with AlGaN/GaN field-effect transistors. Appl. Phys. Lett. 86, 033901 ( 2005).
Ashkenasy, G., Cahen, D., Cohen, R., Shanzer, A. & Vilan, A. Molecular engineering of semiconductor surfaces and devices. Acc. Chem. Res. 35, 121–128 ( 2002).
Luber, S. et al. Liquid phase sensors based on chemically functionalized GaAs/AlGaAs heterostructures. Physica E 21, 1111–1115 ( 2004).
Cui, Y., Wei, Q., Park, H. & Lieber, C. M. Nanowire nanosensors for highly sensitive and selective detection of biological and chemical species. Science 293, 1289–1292 ( 2001).
Saffman, P. G. & Delbrück, M. Brownian motion in biological membranes. Proc. Natl Acad. Sci. USA 72, 3111–3113 ( 1975).
Kühner, M., Tampé, R. & Sackmann, E. Lipid mono- and bilayer supported on polymer films: Composite polymer-lipid films on solid substrates. Biophys. J. 67, 217–226 ( 1994).
Acknowledgements
We thank all our collaborators who contributed to this subject, including S. Kaufmann, O. Purrucker, F. Rehfeldt, A. Wong, M. Tutus, J. Hermann, S. Gönnenwein, M. Schneider, K. Adlkofer, H. Hillebrandt, G. Wiegand and S. Gritsch. We thank the groups of G. Wegner, R. Jordan, L. Moroder, M. Fischer, M. Tornow, M. Eickhoff, M. Stutzmann, G. Abstreiter and S. G. Boxer for fruitful collaborations and inspiring discussion. This work was supported through Deutsche Forschungs Gemeinschaft (DFG), National Science Foundation (NSF-MRSEC) and Fonds der Chemischen Industrie.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Author Information Reprints and permissions information is available at npg.nature.com/reprintsandpermissions.
Rights and permissions
About this article
Cite this article
Tanaka, M., Sackmann, E. Polymer-supported membranes as models of the cell surface. Nature 437, 656–663 (2005). https://doi.org/10.1038/nature04164
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature04164
This article is cited by
-
Spherical Confinement Generates Entropic Force to Accelerate Polymer Chain Detachment
Chinese Journal of Polymer Science (2024)
-
Spatiotemporal stop-and-go dynamics of the mitochondrial TOM core complex correlates with channel activity
Communications Biology (2022)
-
Systematic design of cell membrane coating to improve tumor targeting of nanoparticles
Nature Communications (2022)
-
Model architectures for bacterial membranes
Biophysical Reviews (2022)
-
Critical role of lipid membranes in polarization and migration of cells: a biophysical view
Biophysical Reviews (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.