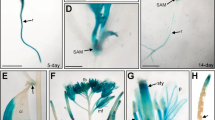
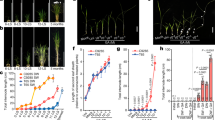
Abstract
Gibberellins (GAs) are phytohormones that are essential for many developmental processes in plants. It has been postulated that plants have both membrane-bound and soluble GA receptors; however, no GA receptors have yet been identified. Here we report the isolation and characterization of a new GA-insensitive dwarf mutant of rice, gid1. The GID1 gene encodes an unknown protein with similarity to the hormone-sensitive lipases, and we observed preferential localization of a GID1–green fluorescent protein (GFP) signal in nuclei. Recombinant glutathione S-transferase (GST)–GID1 had a high affinity only for biologically active GAs, whereas mutated GST–GID1 corresponding to three gid1 alleles had no GA-binding affinity. The dissociation constant for GA4 was estimated to be around 10-7 M, enough to account for the GA dependency of shoot elongation. Moreover, GID1 bound to SLR1, a rice DELLA protein, in a GA-dependent manner in yeast cells. GID1 overexpression resulted in a GA-hypersensitive phenotype. Together, our results indicate that GID1 is a soluble receptor mediating GA signalling in rice.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Davies, P. J. Plant Hormones (Kluwer, Dordrecht, The Netherlands, 1995)
Hedden, P. & Philips, A. L. Gibberellin metabolism. New insights revealed by the genes. Trends Plant Sci. 5, 523–530 (2000)
Sakamoto, T. et al. An overview of gibberellin metabolism enzyme genes and their related mutants in rice. Plant Physiol. 134, 1642–1653 (2004)
Ikeda, A. et al. slender rice, a constitutive gibberellin response mutant, is caused by a null mutation of the SLR1 gene, an ortholog of the height-regulating gene GAI/RGA/RHT/D8. Plant Cell 13, 999–1010 (2001)
Itoh, H., Ueguchi-Tanaka, M., Sato, Y., Ashikari, M. & Matsuoka, M. The gibberellin signalling pathway is regulated by the appearance and disappearance of SLENDER RICE1 in nuclei. Plant Cell 14, 57–70 (2002)
Peng, J. et al. The Arabidopsis GAI gene defines a signalling pathway that negatively regulates gibberellin responses. Genes Dev. 11, 3194–3205 (1997)
Silverstone, A. L., Ciampaglio, C. N. & Sun, T.-P. The Arabidopsis RGA gene encodes a transcriptional regulator repressing the gibberellin signal transduction pathway. Plant Cell 2, 155–169 (1998)
Gubler, F., Chandler, P. M., White, R. G., Llewellyn, D. J. & Jacobsen, J. V. Gibberellin signalling in barley aleurone cells. Control of SLR1 and GAMYB expression. Plant Physiol. 129, 191–200 (2002)
Itoh, H., Matsuoka, M. & Steber, C. M. A role for the ubiquitin-26S-proteasome pathway in gibberellin signalling. Trends Plant Sci. 8, 492–497 (2003)
Sasaki, A. et al. Accumulation of phosphorylated repressor for gibberellin signalling in an F-box mutant. Science 299, 1896–1898 (2003)
Gomi, K. et al. GID2, an F-box subunit of the SCF E3 complex, specifically interacts with phosphorylated SLR1 protein and regulates the gibberellin-dependent degradation of SLR1 in rice. Plant J. 37, 626–634 (2004)
Hooley, R. et al. Gibberellin perception and the Avena Fatua aleurone: do our molecular keys fit the correct locks? Biochem. Soc. Trans. 20, 85–89 (1992)
Lovegrove, A., Barratt, D. H., Beale, M. H. & Hooley, R. Gibberellin-photoaffinity labelling of two polypeptides in plant plasma membranes. Plant J. 15, 311–320 (1998)
Nakajima, M. et al. Partial purification and characterization of a gibberellin-binding protein from seedlings of Azukia angularis. Biochem. Biophys. Res. Commun. 241, 782–786 (1997)
Itoh, H., Ueguchi-Tanaka, M., Sentoku, N., Kitano, H. & Matsuoka, M. Cloning and functional analysis of two gibberellin 3β-hydroxylase genes that are differently expressed during the growth of rice. Proc. Natl Acad. Sci. USA 98, 8909–8914 (2001)
Thornton, T. M., Swain, S. M. & Olszewski, N. E. Gibberellin signal transduction presents ellipsis the SPY who O-GlcNAc'd me. Trends Plant Sci. 4, 424–428 (1999)
Sasaki, A. et al. Green revolution: a mutant gibberellin-synthesis gene in rice. Nature 416, 701–702 (2002)
Itoh, H. et al. Dissection of the phosphorylation of rice DELLA protein, SLENDER RICE1. Plant Cell Physiol. 46, 1392–1399 (2005)
Marchler-Bauer, A. et al. CDD: a Conserved Domain Database for protein classification. Nucleic Acids Res. 33, 192–196 (2005)
Osterlund, T. et al. Domain-structure analysis of recombinant rat hormone-sensitive lipase. Biochem. J. 319, 411–420 (1996)
Manco, G. et al. Cloning, overexpression, and properties of a new thermophilic and thermostable esterase with sequence similarity to hormone-sensitive lipase subfamily from the archaeon Arcaeoglobus fulgidus. Arch. Biochem. Biophys. 373, 182–192 (2000)
Osterlund, T. Structure-function relationships of hormone-sensitive lipase. Eur. J. Biochem. 268, 1899–1907 (2001)
Nishijima, T., Koshioka, M. & Yamazaki, H. Use of several gibberellin biosynthesis inhibitors in sensitized rice seedling bioassays. Biosci. Biotech. Biochem. 58, 572–573 (1994)
Nakayama, I. et al. Effects of a new plant growth regulator prohexadine calcium (BX-112) on shoot elongation caused by exogenously applied gibberellins in rice (Oryza sativa L.) seedlings. Plant Cell Physiol. 31, 195–200 (1990)
Natsume, T., Hirota, J., Yoshikawa, F., Furuichi, T. & Mikoshiba, K. Real time analysis of interaction between inositol 1,4,5-triphosphatereceptor type I and its ligand. Biochem. Biophys. Res. Commun. 260, 527–533 (1999)
Ueguchi-Tanaka, M. et al. Rice dwarf mutant d1, which is defective in the α subunit of the heterotrimeric G protein, affects gibberellin signal transduction. Proc. Natl Acad. Sci. USA 97, 11638–11643 (2000)
Dharmasiri, N., Dharmasiri, S. & Estelle, M. The F-box protein TIR1 is an auxin receptor. Nature 435, 441–445 (2005)
Keinski, S. & Leyser, O. The Arabidopsis F-box protein TIR1 is an auxin receptor. Nature 435, 446–451 (2005)
Hiei, Y., Ohta, S., Komari, T. & Kumashiro, T. Efficient transformation of rice (Oryza Sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J. 6, 270–282 (1994)
Nishimura, A., Ito, M., Kamiya, N., Sato, Y. & Matsuoka, M. OsPNH1 regulates leaf development and maintenance of the shoot apical meristem in rice. Plant J. 30, 189–201 (2002)
Kobayashi, M. et al. Fluctuation of endogenous gibberellin and abscisic acid levels in the germinating seeds of barley. Biosci. Biotechnol. Biochem. 59, 1969–1970 (1995)
Acknowledgements
We thank H. Oomiya, S. Hattori and I. Aichi for technical assistance, and C. Ueguchi for suggestions regarding the yeast two-hybrid assay. This work was supported in part by a Grant-in-Aid for the Center of Excellence, the Program for the Promotion of Basic Research Activities for Innovative Bioscience (M.M. and H.K), the MAFF Rice Genome Project, IP1003 (M.A. and M.M.), and by the Ministry of Education, Culture, Sports, Science and Technology of Japan (I.Y., M.N. and M.U.-T.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Sequence data from this article have been deposited in the DDBJ/EMBL/GenBank databases under accession number AB211399. Reprints and permissions information is available at npg.nature.com/reprintsandpermissions. The authors declare no competing financial interests.
Supplementary information
Supplementary Figure S1
Positional cloning and the structure of GID1 gene. (PDF 220 kb)
Supplementary Figure S2a
Reversibility of GA-binding to GST-GID1. (PDF 248 kb)
Supplementary Figure S3
Structures of GAs in Table1. (PDF 227 kb)
Supplementary Figure Legends
Text to accompany the above Supplementary Figures (DOC 22 kb)
Rights and permissions
About this article
Cite this article
Ueguchi-Tanaka, M., Ashikari, M., Nakajima, M. et al. GIBBERELLIN INSENSITIVE DWARF1 encodes a soluble receptor for gibberellin. Nature 437, 693–698 (2005). https://doi.org/10.1038/nature04028
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature04028
This article is cited by
-
Uncovering the Genomic Regions Associated with Yield Maintenance in Rice Under Drought Stress Using an Integrated Meta-Analysis Approach
Rice (2024)
-
Gibberellin-mediated far-red light-induced leaf expansion in cucumber seedlings
Protoplasma (2024)
-
Uncovering the involvement of DoDELLA1-interacting proteins in development by characterizing the DoDELLA gene family in Dendrobium officinale
BMC Plant Biology (2023)
-
Transcriptome analysis during vernalization in wheat (Triticum aestivum L.)
BMC Genomic Data (2023)
-
Characterizing the circadian connectome of Ocimum tenuiflorum using an integrated network theoretic framework
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.