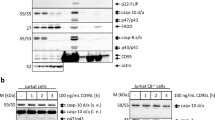
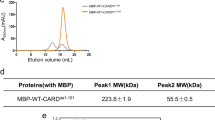
Abstract
Apoptosis is executed by caspases, which undergo proteolytic activation in response to cell death stimuli1. The apoptotic protease-activating factor 1 (Apaf-1) controls caspase activation downstream of mitochondria2. During apoptosis, Apaf-1 binds to cytochrome c and in the presence of ATP/dATP forms an apoptosome, leading to the recruitment and activation of the initiator caspase, caspase-9 (ref. 2). The mechanisms underlying Apaf-1 function are largely unknown. Here we report the 2.2-Å crystal structure of an ADP-bound, WD40-deleted Apaf-1, which reveals the molecular mechanism by which Apaf-1 exists in an inactive state before ATP binding. The amino-terminal caspase recruitment domain packs against a three-layered α/β fold, a short helical motif and a winged-helix domain, resulting in the burial of the caspase-9-binding interface. The deeply buried ADP molecule serves as an organizing centre to strengthen interactions between these four adjoining domains, thus locking Apaf-1 in an inactive conformation. Apaf-1 binds to and hydrolyses ATP/dATP and their analogues. The binding and hydrolysis of nucleotides seem to drive conformational changes that are essential for the formation of the apoptosome and the activation of caspase-9.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Riedl, S. J. & Shi, Y. Molecular mechanisms of caspase regulation during apoptosis. Nature Rev. Mol. Cell Biol. 5, 897–907 (2004)
Wang, X. The expanding role of mitochondria in apoptosis. Genes Dev. 15, 2922–2933 (2001)
Li, P. et al. Cytochrome c and dATP-dependent formation of Apaf-1/Caspase-9 complex initiates an apoptotic protease cascade. Cell 91, 479–489 (1997)
Zou, H., Li, Y., Liu, X. & Wang, X. An APAF-1-cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. J. Biol. Chem. 274, 11549–11556 (1999)
Jiang, X. & Wang, X. Cytochrome c promotes caspase-9 activation by inducing nucleotide binding to Apaf-1. J. Biol. Chem. 275, 31199–31203 (2000)
Hu, Y., Benedict, M. A., Ding, L. & Nunez, G. Role of cytochrome c and dATP/ATP hydrolysis in Apaf-1-mediated caspase-9 activation and apoptosis. EMBO J. 18, 3586–3595 (1999)
Saleh, A., Srinivasula, S. M., Acharya, S., Fishel, R. & Alnemri, E. S. Cytochrome c and dATP-mediated oligomerization of Apaf-1 is a prerequisite for procaspase-9 activation. J. Biol. Chem. 274, 17941–17945 (1999)
Rodriguez, J. & Lazebnik, Y. Caspase-9 and Apaf-1 form an active holoenzyme. Genes Dev. 13, 3179–3184 (1999)
Inohara, N. & Nunez, G. The NOD: a signaling module that regulates apoptosis and host defense against pathogens. Oncogene 20, 6473–6481 (2001)
Hu, Y., Ding, L., Spencer, D. M. & Nunez, G. WD-40 repeat region regulates Apaf-1 self-association and procaspase-9 activation. J. Biol. Chem. 273, 33489–33494 (1998)
Srinivasula, S. M., Ahmad, M., Fernandes-Alnemri, T. & Alnemri, E. S. Autoactivation of procaspase-9 by Apaf-1-mediated oligomerization. Mol. Cell 1, 949–957 (1998)
Kaufmann, E. & Knochel, W. Five years on the wings of fork head. Mech. Dev. 57, 3–20 (1996)
Holm, L. & Sander, C. Protein structure comparison by alignment of distance matrices. J. Mol. Biol. 233, 123–138 (1993)
Lenzen, C. U., Steinmann, D., Whiteheart, S. W. & Weis, W. I. Crystal structure of the hexamerization domain of N-ethylmaleimide-sensitive fusion protein. Cell 94, 525–536 (1998)
Zhang, X. et al. Structure of the AAA ATPase p97. Mol. Cell 6, 1473–1484 (2000)
Lupas, A. N. & Martin, J. AAA proteins. Curr. Opin. Struct. Biol. 12, 746–753 (2002)
Jaroszewski, L., Rychlewski, L., Reed, J. C. & Godzik, A. ATP-activated oligomerization as a mechanism for apoptosis regulation: fold and mechanism prediction for CED-4. Proteins 39, 197–203 (2000)
Qin, H. et al. Structural basis of procaspase-9 recruitment by the apoptotic protease-activating factor 1. Nature 399, 547–555 (1999)
Genini, D. et al. Nucleotide requirements for the in vitro activation of the apoptosis protein-activating factor-1-mediated caspase pathway. J. Biol. Chem. 275, 29–34 (2000)
Leoni, L. M. et al. Induction of an apoptotic program in cell-free extracts by 2-chloro-2′-deoxyadenosine 5′-triphosphate and cytochrome c. Proc. Natl Acad. Sci. USA 95, 9567–9571 (1998)
Cain, K., Brown, D. G., Langlais, C. & Cohen, G. M. Caspase activation involves the formation of the aposome, a large (∼ 700 kDa) caspase-activating complex. J. Biol. Chem. 274, 22686–22692 (1999)
Bochtler, M. et al. The structures of HsIU and the ATP-dependent protease HsIU-HsIV. Nature 403, 800–805 (2000)
Gai, D., Zhao, R., Li, D., Finkielstein, C. V. & Chen, X. S. Mechanisms of conformational change for a replicative hexameric helicase of SV40 large tumor antigen. Cell 119, 47–60 (2004)
Acehan, D. et al. Three-dimensional structure of the apoptosome: Implications for assembly, procaspase-9 binding and activation. Mol. Cell 9, 423–432 (2002)
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997)
Terwilliger, T. C. & Berendzen, J. Automated structure solution for MIR and MAD. Acta Crystallogr. D 55, 849–861 (1999)
Jones, T. A., Zou, J.-Y., Cowan, S. W. & Kjeldgaard, M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr. A 47, 110–119 (1991)
Collaborative Computational Project No 4. The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D 50, 760–763 (1994)
Kraulis, P. J. Molscript: a program to produce both detailed and schematic plots of protein structures. J. Appl. Crystallogr. 24, 946–950 (1991)
Nicholls, A., Sharp, K. A. & Honig, B. Protein folding and association: insights from the interfacial and thermodynamic properties of hydrocarbons. Proteins Struct. Funct. Genet. 11, 281–296 (1991)
Acknowledgements
We thank M. Becker, A. Saxena, K. Dedrick and L. Walsh for assistance, A. Wist for help with the TLC, L. Gu for help with the omit map, F. Hughson for critically reading the manuscript, and members of the Shi laboratory for discussion. This work was supported by the NIH. S.J.R. is a Fellow of the Leukemia and Lymphoma Society.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Supplementary information
Supplementary Figure S1
Sequence alignment of the human Apaf-1 protein with its homologues in fish, fly and worm (CED-4). (JPG 492 kb)
Supplementary Figure S2
Apaf-1 can activate caspase-9 in a 1:1 complex. a, Apaf-1 is capable of binding to caspase-9 in the absence of ATP/dATP. (JPG 38 kb)
Supplementary Table S1
Diffraction data and refinement statistics. (DOC 22 kb)
Supplementary Figure Legends
Legends to accompany Supplementary Figures S1 and S2. (DOC 20 kb)
Rights and permissions
About this article
Cite this article
Riedl, S., Li, W., Chao, Y. et al. Structure of the apoptotic protease-activating factor 1 bound to ADP. Nature 434, 926–933 (2005). https://doi.org/10.1038/nature03465
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature03465
This article is cited by
-
The NLR gene family: from discovery to present day
Nature Reviews Immunology (2023)
-
Structural basis of NLR activation and innate immune signalling in plants
Immunogenetics (2022)
-
The APAF1_C/WD40 repeat domain-encoding gene from the sea lettuce Ulva mutabilis sheds light on the evolution of NB-ARC domain-containing proteins in green plants
Planta (2022)
-
Mitochondrial functions and melatonin: a tour of the reproductive cancers
Cellular and Molecular Life Sciences (2019)
-
Molecular changes in solitary fibrous tumor progression
Journal of Molecular Medicine (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.