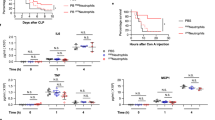
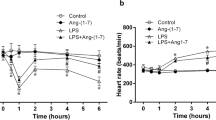
Abstract
Endothelin-1 (ET-1) is a 21-amino-acid peptide, derived from vascular endothelial cells, with potent vasoconstrictor activity1. ET-1 has been implicated in diverse physiological or pathological processes2,3, including the vascular changes associated with sepsis2,3,4,5. However, the factors that regulate ET-1-associated toxicity during bacterial infections, or in other settings, are not fully understood2,3,4,5. Both the pathology associated with certain allergic and autoimmune disorders6,7, and optimal host defence against bacterial and parasitic infections8,9,10 are mediated by mast cells. In vitro, mast cells can produce ET-1 (ref. 11), undergo ET-1-dependent and endothelin-A receptor (ETA)-dependent activation12,13, and release proteases that degrade ET-1 (ref. 14). Although the potential relationships between mast cells and the ET-1 system thus may be complex, the importance of interactions between ET-1 and mast cells in vivo is obscure. Here we show that ETA-dependent mast-cell activation can diminish both ET-1 levels and ET-1-induced pathology in vivo, and also can contribute to optimal survival during acute bacterial peritonitis. These findings identify a new biological function for mast cells: promotion of homeostasis by limiting the toxicity associated with an endogenous mediator.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Yanagisawa, M. et al. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 332, 411–415 (1988)
Wanecek, M., Weitzberg, E., Rudehill, A. & Oldner, A. The endothelin system in septic and endotoxin shock. Eur. J. Pharmacol. 407, 1–15 (2000)
Kedzierski, R. M. & Yanagisawa, M. Endothelin system: the double-edged sword in health and disease. Annu. Rev. Pharmacol. Toxicol. 41, 851–876 (2001)
Szalay, L., Kaszaki, J., Nagy, S. & Boros, M. The role of endothelin-1 in circulatory changes during hypodynamic sepsis in the rat. Shock 10, 123–128 (1998)
Ornan, D. A., Chaudry, I. H. & Wang, P. The dissociation between upregulated endothelins and hemodynamic responses during polymicrobial sepsis. Biochim. Biophys. Acta 1501, 211–218 (2000)
Kawakami, T. & Galli, S. J. Regulation of mast-cell and basophil function and survival by IgE. Nature Rev. Immunol. 2, 773–786 (2002)
Benoist, C. & Mathis, D. Mast cells in autoimmune disease. Nature 420, 875–878 (2002)
Echtenacher, B., Männel, D. N. & Hültner, L. Critical protective role of mast cells in a model of acute septic peritonitis. Nature 381, 75–77 (1996)
Malaviya, R., Ikeda, T., Ross, E. & Abraham, S. N. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-alpha. Nature 381, 77–80 (1996)
Galli, S. J., Chatterjea, D. & Tsai, M. in The Innate Immune Response to Infection (ed. Gordon, S.) 111–132 (ASM, Berlin, 2004)
Ehrenreich, H. et al. Endothelins belong to the assortment of mast cell-derived and mast cell-bound cytokines. New Biol. 4, 147–156 (1992)
Yamamura, H., Nabe, T., Kohno, S. & Ohata, K. Endothelin-1, one of the most potent histamine releasers in mouse peritoneal mast cells. Eur. J. Pharmacol. 265, 9–15 (1994)
Yamamura, H., Nabe, T., Kohno, S. & Ohata, K. Mechanism of histamine release by endothelin-1 distinct from that by antigen in mouse bone marrow-derived mast cells. Eur. J. Pharmacol. 288, 269–275 (1995)
Metsärinne, K. P. et al. Activated mast cells increase the level of endothelin-1 mRNA in cocultured endothelial cells and degrade the secreted peptide. Arterioscler. Thromb. Vasc. Biol. 22, 268–273 (2002)
Kitamura, Y., Go, S. & Hatanaka, K. Decrease of mast cells in W/Wv mice and their increase by bone marrow transplantation. Blood 52, 447–452 (1978)
Galli, S. J., Zsebo, K. M. & Geissler, E. N. The kit ligand, stem cell factor. Adv. Immunol. 55, 1–96 (1994)
Tsai, M., Tam, S. Y., Wedemeyer, J. & Galli, S. J. Mast cells derived from embryonic stem cells: a model system for studying the effects of genetic manipulations on mast cell development, phenotype, and function in vitro and in vivo. Int. J. Hematol. 75, 345–349 (2002)
Nakano, T. et al. Fate of bone marrow-derived cultured mast cells after intracutaneous, intraperitoneal, and intravenous transfer to genetically mast cell-deficient W/Wv mice. Evidence that cultured mast cells can give rise to both connective tissue and mucosal mast cells. J. Exp. Med. 162, 1025–1043 (1985)
Maurer, M. et al. The c-kit ligand, stem cell factor, can enhance innate immunity through effects on mast cells. J. Exp. Med. 188, 2343–2348 (1998)
Boesiger, J. et al. Mast cells can secrete vascular permeability factor/vascular endothelial cell growth factor and exhibit enhanced release after immunoglobulin E-dependent upregulation of Fcɛ receptor I expression. J. Exp. Med. 188, 1135–1145 (1998)
Maguire, J. J., Kuc, R. E. & Davenport, A. P. Vasoconstrictor activity of novel endothelin peptide, ET-1(1–31), in human mammary and coronary arteries in vitro. Br. J. Pharmacol. 134, 1360–1366 (2001)
Opgenorth, T. J. Endothelin receptor antagonism. Adv. Pharmacol. 33, 1–64 (1995)
Clouthier, D. E., Williams, S. C., Hammer, R. E., Richardson, J. A. & Yanagisawa, M. Cell-autonomous and nonautonomous actions of endothelin-A receptor signaling in craniofacial and cardiovascular development. Dev. Biol. 261, 506–519 (2003)
Tsai, M. et al. In vivo immunological function of mast cells derived from embryonic stem cells: an approach for the rapid analysis of even embryonic lethal mutations in adult mice in vivo. Proc. Natl Acad. Sci. USA 97, 9186–9190 (2000)
Prodeus, A. P., Zhou, X., Maurer, M., Galli, S. J. & Carroll, M. C. Impaired mast cell-dependent natural immunity in complement C3-deficient mice. Nature 390, 172–175 (1997)
Ahn, G. Y. et al. The expression of endothelin-1 and its binding sites in mouse skin increased after ultraviolet B irradiation or local injection of tumor necrosis factor alpha. J. Dermatol. 25, 78–84 (1998)
Sugiura, M., Inagami, T. & Kon, V. Endotoxin stimulates endothelin-release in vivo and in vitro as determined by radioimmunoassay. Biochem. Biophys. Res. Commun. 161, 1220–1227 (1989)
Lundblad, R. & Giercksky, K. E. Endothelin concentrations in experimental sepsis: profiles of big endothelin and endothelin 1–21 in lethal peritonitis in rats. Eur. J. Surg. 161, 9–16 (1995)
Boros, M., Szalay, L. & Kaszaki, J. Endothelin-1 induces mucosal mast cell degranulation and tissue injury via ETA receptors. Clin. Sci. (Lond.) 103(suppl. 48), 31S–34S (2002)
Higginbotham, R. D. Mast cells and local resistance to Russell's viper venom. J. Immunol. 95, 867–875 (1965)
Acknowledgements
We thank R. Parker of the Biometrics Center of the Beth Israel Deaconess Medical Center for consultation regarding the statistical analysis of the data, S. Fish, L. Fox, M. Liebersbach and A. Xu for technical assistance, M.-H. Jouvin for discussions and critical reading of the manuscript, and R. Paus for supporting M. Metz. This work was supported by United States Public Health Science Grants (to S.J.G.), by grants of the Deutsche Forschungsgemeinschaft (to M. Maurer, J.W. and M. Metz), and by a grant of the Boehringer Ingelheim Fonds (to M. Metz).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Supplementary information
Supplementary Figure 1
ET-1, but not a scrambled peptide, induces hypothermia in mast cell-deficient KitW/KitW-v mice. (PDF 17 kb)
Supplementary Figure 2
Co-incubation with mast cells ex vivo reduces the toxicity of ET-1 in vivo. (PDF 18 kb)
Supplementary Figure 3
Mast cells degrade ET-1 in vitro in a time- and dose-dependent manner. (PDF 40 kb)
Supplementary Figure 4
ET-1 induced degranulation. (PDF 52 kb)
Supplementary Figure 5
Pharmacological evidence that the ability of peritoneal mast cells to reduce the toxicity of ET-1 reflects their expression of ETA. (PDF 23 kb)
Supplementary Figure 6
Extent of PMC degranulation in Kit+/+ mice. (PDF 10 kb)
Supplementary Figure 7
In aperitoneal levels of endogenous ET-1 in female Kit+/+ mice.tr (PDF 11 kb)
Supplementary Figure 8
Pharmacological evidence that ET-1 contributes to mortality after CLP via ETB. (PDF 18 kb)
Rights and permissions
About this article
Cite this article
Maurer, M., Wedemeyer, J., Metz, M. et al. Mast cells promote homeostasis by limiting endothelin-1-induced toxicity. Nature 432, 512–516 (2004). https://doi.org/10.1038/nature03085
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature03085
This article is cited by
-
Microinjection of pruritogens in NGF-sensitized human skin
Scientific Reports (2021)
-
Mouse connective tissue mast cell proteases tryptase and carboxypeptidase A3 play protective roles in itch induced by endothelin-1
Journal of Neuroinflammation (2020)
-
Topical application of endothelin receptor A antagonist attenuates imiquimod-induced psoriasiform skin inflammation
Scientific Reports (2020)
-
Mast cells and IgE in defense against lethality of venoms: Possible "benefit" of allergy*
Allergo Journal (2020)
-
Mast cells and IgE in defense against lethality of venoms: Possible “benefit” of allergy
Allergo Journal International (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.