Abstract
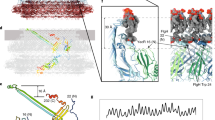
The bacterial flagellum is a motile organelle, and the flagellar hook is a short, highly curved tubular structure that connects the flagellar motor to the long filament acting as a helical propeller. The hook is made of about 120 copies of a single protein, FlgE, and its function as a nano-sized universal joint is essential for dynamic and efficient bacterial motility and taxis. It transmits the motor torque to the helical propeller over a wide range of its orientation for swimming and tumbling. Here we report a partial atomic model of the hook obtained by X-ray crystallography of FlgE31, a major proteolytic fragment of FlgE lacking unfolded terminal regions, and by electron cryomicroscopy and three-dimensional helical image reconstruction of the hook. The model reveals the intricate molecular interactions and a plausible switching mechanism for the hook to be flexible in bending but rigid against twisting for its universal joint function.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Macnab, R. M. How bacteria assemble flagella. Annu. Rev. Microbiol. 57, 77–100 (2003)
Berg, H. C. The rotary motor of bacterial flagella. Annu. Rev. Biochem. 72, 19–54 (2003)
Kojima, S. & Blair, D. The bacterial flagellar motor: structure and function of a complex molecular machine. Int. Rev. Cytol. 233, 93–134 (2004)
DePamphilis, M. L. & Adler, J. Purification of intact flagella from Escherichia coli and Bacillus subtilis. J. Bacteriol. 105, 376–383 (1971)
DePamphilis, M. L. & Adler, J. Fine structure and isolation of the hook–basal body complex of flagella from Escherichia coli and Bacillus subtilis. J. Bacteriol. 105, 384–395 (1971)
Berg, H. C. & Anderson, R. A. Bacteria swim by rotating their flagellar filaments. Nature 245, 380–382 (1973)
Silverman, M. & Simon, M. Flagellar rotation and the mechanism of bacterial motility. Nature 249, 73–74 (1974)
Namba, K. & Vonderviszt, F. Molecular architecture of bacterial flagellum. Q. Rev. Biophys. 30, 1–65 (1997)
Macnab, R. M. & Ornston, M. K. Normal-to-curly flagellar transitions and their role in bacterial tumbling. Stabilization of an alternative quaternary structure by mechanical force. J. Mol. Biol. 112, 1–30 (1977)
Turner, L., Ryu, W. S. & Berg, H. C. Real-time imaging of fluorescent flagellar filaments. J. Bacteriol. 182, 2793–2801 (2000)
Wagenknecht, T., DeRosier, D. J., Aizawa, S.-I. & Macnab, R. M. Flagellar hook structures of Caulobacter and Salmonella and their relationship to filament structure. J. Mol. Biol. 162, 69–87 (1982)
Kagawa, H., Aizawa, S. I. & Asakura, S. Transformations in isolated polyhooks. J. Mol. Biol. 129, 333–336 (1979)
Williams, A. W. et al. Mutation in fliK and flhB affecting flagellar hook and filament assembly in Salmonella typhimurium. J. Bacteriol. 178, 2960–2970 (1996)
Hirano, T., Yamaguchi, S., Oosawa, K. & Aizawa, S.-I. Roles of FliK and FlhB in the determination of flagellar hook length in Salmonella typhimurium. J. Bacteriol. 176, 5439–5449 (1994)
Wagenknecht, T., DeRosier, D. J., Shapiro, L. & Weissborn, A. Three-dimensional reconstruction of the flagellar hook from Caulobacter crescentus. J. Mol. Biol. 151, 439–465 (1981)
Kutsukake, K., Suzuki, T., Yamaguchi, S. & Iino, T. Role of gene flaFV on flagellar hook formation in Salmonella typhimurium. J. Bacteriol. 140, 267–275 (1979)
Aizawa, S. & Maeda, Y. A new method for determination of parity in optical diffraction patterns from the structures with helical symmetry. J. Mol. Biol. 137, 437–442 (1980)
Kato, S., Okamoto, M. & Asakura, S. Polymorphic transition of the flagellar polyhook from Escherichia coli and Salmonella typhimurium. J. Mol. Biol. 173, 463–476 (1984)
Morgan, D. G., Macnab, R. M., Francis, N. R. & DeRosier, D. J. Domain organization of the subunit of the Salmonella typhimurium flagellar hook. J. Mol. Biol. 229, 79–84 (1993)
Homma, M., DeRosier, D. J. & Macnab, R. M. Flagellar hook and hook-associated proteins of Salmonella typhimurium and their relationship to other axial components of the flagellum. J. Mol. Biol. 213, 819–832 (1990)
Samatey, F. A. et al. Structure of the bacterial flagellar protofilament and implications for a switch for supercoiling. Nature 410, 331–337 (2001)
Samatey, F. A., Imada, K., Vonderviszt, F., Shirakihara, Y. & Namba, K. Crystallization of the F41 fragment of flagellin and data collection from extremely thin crystals. J. Struct. Biol. 132, 106–111 (2000)
Vonderviszt, F., Ishima, R., Akasaka, K. & Aizawa, S. Terminal disorder: a common structural feature of the axial proteins of bacterial flagellum? J. Mol. Biol. 226, 575–579 (1992)
Vonderviszt, F., Zavodszky, P., Ishimura, M., Uedaira, H. & Namba, K. Structural organization and assembly of flagellar hook protein from Salmonella typhimurium. J. Mol. Biol. 251, 520–532 (1995)
Holm, L. & Sander, C. Protein structure comparison by alignment of distance matrices. J. Mol. Biol. 233, 123–138 (1993)
Yonekura, K., Maki-Yonekura, S. & Namba, K. Complete atomic model of the bacterial flagellar filament by electron cryomicroscopy. Nature 424, 643–650 (2003)
Chen, J. Z., Fürst, J., Chapman, M. S. & Grigorieff, N. Low resolution structure refinement in electron microscopy. J. Struct. Biol. 144, 144–151 (2003)
Hasegawa, K., Yamashita, I. & Namba, K. Quasi- and nonequivalence in the structure of bacterial flagellar filament. Biophys. J. 74, 569–575 (1998)
Lowe, G., Meister, M. & Berg, H. C. Rapid rotation of flagellar bundles in swimming bacteria. Nature 325, 637–640 (1987)
Kudo, S., Magariyama, Y. & Aizawa, S. Abrupt changes in flagellar rotation observed by laser dark-field microscopy. Nature 346, 677–680 (1990)
Yamashita, I. et al. Structure and switching of bacterial flagellar filaments studied by X-ray fiber diffraction. Nature Struct. Biol. 5, 125–132 (1998)
Fahrner, K. A., Block, S. M., Krishnaswamy, S., Parkinson, J. S. & Berg, H. C. A mutant hook-associated protein (HAP3) facilitates torsionally induced transformations of the flagellar filament of Escherichia coli. J. Mol. Biol. 238, 173–186 (1994)
Samatey, F. A., Matsunami, H., Imada, K., Nagashima, S. & Namba, K. Crystallization of a core fragment of the hook protein FlgE. Acta Crystallogr. D (in the press)
Otwinowski, Z. & Minor, W. Processing of X-ray Diffraction Data Collected in Oscillation Mode (Academic, New York, 1997)
Powell, H. R. The Rossmann Fourier autoindexing algorithm in MOSFLM. Acta Crystallogr. D 55, 1690–1695 (1999)
Collaborative Computational Project No. 4, The CCP4 suite: Programs for protein crystallography. Acta Crystallogr. D 50, 760–763 (1994)
Terwilliger, T. C. & Berendzen, J. Automated structure solution for MIR and MAD. Acta Crystallogr. D 55, 849–861 (1999)
Terwilliger, T. C. Maximum-likelihood density modification. Acta Crystallogr. D 56, 965–972 (2000)
Perrakis, A., Morris, R. & Lamzin, V. S. Automated protein model building combined with iterative structure refinement. Nature Struct. Biol. 6, 458–463 (1999)
Jones, T. A., Zhou, J. Y., Cowan, S. W. & Kjeldgaard, M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr. A 47, 110–119 (1991)
Murshudov, G. N., Vagin, A. A. & Dodson, E. J. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D 53, 240–255 (1997)
Francis, N. R., Sosinsky, G. E., Thomas, D. & DeRosier, D. J. Isolation, characterization and structure of bacterial flagellar motors containing the switch complex. J. Mol. Biol. 235, 1261–1270 (1994)
Morgan, D. G., Owen, C., Melanson, L. A. & DeRosier, D. J. Structure of bacterial flagellar filaments at 11 Å resolution: packing of the alpha-helices. J. Mol. Biol. 249, 88–110 (1995)
Case, D. A., et al. AMBER7. (Univ. California, San Francisco, California, 2002)
Kraulis, P. J. MOLSCRIPT: a program to produce both detailed and schematic plots of protein structures. J. Appl. Crystallogr. 24, 946–950 (1991)
Merritt, E. A. & Bacon, D. J. Raster3D: Photorealistic molecular graphics. Methods Enzymol. 277, 505–524 (1997)
Acknowledgements
We thank R. M. Macnab, who passed away suddenly in September 2003, for his invaluable discussion on the structure and function of the flagellar hook; the staff members of beamline ID29 at the European Synchrotron Radiation Facility (ESRF) in Grenoble and beamline BL41XU at the 8 GeV Super Photon ring (SPring-8) in Harima for their help for the data collection; and F. Oosawa and S. Asakura for continuous support and encouragement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Supplementary information
Supplementary Table 1
Regions involved in intersubunit interactions, listing amino acid residues of FlgE31 involved in the interactions along the 11-start, 5-start and 6-start directions of the hook structure. (DOC 35 kb)
Supplementary Table 2
Summary of refinement statistics of the X-ray crystal structure analysis of FlgE31. (DOC 41 kb)
Supplementary Video 1
Rolling rotation (or “smoke-ring” rotation) of an atomic model of coiled hook during its function as a universal joint. The atomic model is the one shown in Figure 5. During the rolling rotation, each protofilament goes through extension and compression with every revolution. (MOV 13182 kb)
Supplementary Video 2
Possible conformational changes of the protofilaments and subunits on the longitudinal section of the tube wall of the coiled hook during its rolling rotation (four protofilaments have been removed). The inner surface of the layer made of D1 domains can also be seen. (MOV 11056 kb)
Rights and permissions
About this article
Cite this article
Samatey, F., Matsunami, H., Imada, K. et al. Structure of the bacterial flagellar hook and implication for the molecular universal joint mechanism. Nature 431, 1062–1068 (2004). https://doi.org/10.1038/nature02997
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature02997
This article is cited by
-
An overview of the structure and function of the flagellar hook FlgE protein
World Journal of Microbiology and Biotechnology (2023)
-
Role of the flagellar hook in the structural development and antibiotic tolerance of Pseudomonas aeruginosa biofilms
The ISME Journal (2022)
-
Structure of the bacterial flagellar hook cap provides insights into a hook assembly mechanism
Communications Biology (2021)
-
Rapid detection of flagellated and non-flagellated Salmonella by targeting the common flagellar hook gene flgE
Applied Microbiology and Biotechnology (2020)
-
Torque transmission mechanism of the curved bacterial flagellar hook revealed by cryo-EM
Nature Structural & Molecular Biology (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.