Abstract
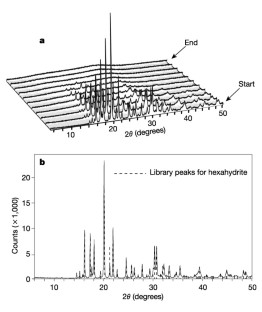
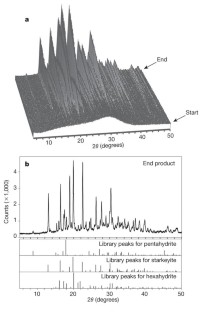
Recent reports of ∼30 wt% of sulphate within saline sediments on Mars1,2—probably occurring in hydrated form3—suggest a role for sulphates in accounting for equatorial H2O observed in a global survey by the Odyssey spacecraft4. Among salt hydrates likely to be present3, those of the MgSO4·nH2O series have many hydration states. Here we report the exposure of several of these phases to varied temperature, pressure and humidity to constrain their possible H2O contents under martian surface conditions. We found that crystalline structure and H2O content are dependent on temperature–pressure history, that an amorphous hydrated phase with slow dehydration kinetics forms at <1% relative humidity, and that equilibrium calculations may not reflect the true H2O-bearing potential of martian soils. Mg sulphate salts can retain sufficient H2O to explain a portion of the Odyssey observations5. Because phases in the MgSO4·nH2O system are sensitive to temperature and humidity, they can reveal much about the history of water on Mars. However, their ease of transformation implies that salt hydrates collected on Mars will not be returned to Earth unmodified, and that accurate in situ analysis is imperative.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
MER Rover web site. 〈http://www.jpl.nasa.gov/mer2004/rover-images/mar-02-2004/images-3-2-04.html〉 (March 2004).
Brueckner, J. Determination of chemical composition of soils and rocks at the MER landing sites Gusev crater and Meridiani Planum using the APXS. Eos 85(17, Jt. Assem. Suppl.), abstract V11A–05 (2004)
Clark, B. C. Implications of abundant hygroscopic minerals in the martian regolith. Icarus 34, 645–665 (1978)
Feldman, W. C. et al. The global distribution of near-surface hydrogen on Mars. J. Geophys. Res. 109, doi:10.1029/2003JE002160 (2004)
Feldman, W. C. et al. Hydrated states of MgSO4 at equatorial latitudes on Mars. Geophys. Res. Lett. 31, doi:10.1029/2004GL020181 (2004)
Clark, B. C. et al. Inorganic analyses of martian surface samples at the Viking landing sites. Science 194, 1283–1288 (1976)
Toulmin, P. III et al. Geochemical and mineralogical interpretation of the Viking inorganic chemical results. J. Geophys. Res. 82, 4625–4634 (1977)
Wänke, H., Brückner, J., Dreibus, G., Rieder, R. & Ryabchikov, I. Chemical composition of rocks and soils at the Pathfinder site. Space Sci. Rev. 96, 317–330 (2001)
Foley, C. N., Economou, T. & Clayton, R. N. Final chemical results from the Mars Pathfinder alpha proton X-ray spectrometer. J. Geophys. Res. 108, doi:10.1029/2002JE002019 (2003)
McSween, H. Y. et al. Basaltic rocks analyzed by the Spirit Rover in Gusev Crater. Science 305, 842–845 (2004)
Christensen, P. R. Martian dust mantling and surface composition: Interpretation of thermophysical properties. J. Geophys. Res. 87, 9985–9998 (1982)
Gendrin, A. & Mustard, J. F. Sulfate-cemented soils detected in TES data through the application of an automatic band detection algorithm. Lunar Planet. Sci. XXXV, abstract 1205 (2004)
Clark, B. C. & Baird, A. K. Is the martian lithosphere sulphur rich? J. Geophys. Res. 84, 8395–8403 (1979)
Banin, A., Han, F. X. & Cicelsky, A. Acidic volatiles and the Mars soil. J. Geophys. Res. 102, 13341–13356 (1997)
Mellon, M. T. & Jakosky, B. M. The distribution and behavior of martian ground ice during past and present epochs. J. Geophys. Res. 100, 11781–11799 (1995)
Bish, D. L., Carey, J. W., Vaniman, D. T. & Chipera, S. J. Stability of hydrous minerals on the martian surface. Icarus 164, 96–103 (2003)
Bish, D. L., Vaniman, D. T., Fialips, C., Carey, J. W. & Feldman, W. C. Can hydrous minerals account for the observed mid-latitude water on Mars? Sixth Int. Conf. on Mars, abstract 3066 (2003)
Clark, B. C. & Van Hart, D. C. The salts of Mars. Icarus 45, 370–378 (1981)
Chou, I.-M. & Seal, R. R. II Determination of epsomite-hexahydrite equilibria by the humidity-buffer technique at 0.1 MPa with implications for phase equilibria in the system MgSO4-H2O. Astrobiology 3, 619–630 (2003)
Hawthorne, F. C., Krivovichev, S. V. & Burns, P. C. in Sulfate Minerals: Crystallography, Geochemistry, and Environmental Significance (eds Alpers, C. N., Jambor, J. L. & Nordstrom, D. K.) 1–112 (Min. Soc. Am., Washington DC, 2000)
Chipera, S. J., Carey, J. W. & Bish, D. L. Controlled-humidity XRD analyses: Application to the study of smectite expansion/contraction. Adv. X-ray Anal. 39, 713–722 (1997)
Savijarvi, H. Mars boundary layer modeling: Diurnal moisture cycle and soil properties at the Viking Lander 1 site. Icarus 117, 120–127 (1995)
Gounelle, M. & Zolensky, M. E. A terrestrial origin for sulfate veins in CI1 chondrites. Meteorit. Planet. Sci. 36, 1321–1329 (2001)
Taylor, L. A., Mao, H. K. & Bell, P. M. “Rust” in the Apollo 16 rocks. Proc. 4th Lunar Sci. Conf. 829–839 (1973)
Acknowledgements
This research was supported by Los Alamos National Laboratory – Directed Research and Development Funding and by a NASA Mars Fundamental Research Program grant. Comments and suggestions by J. F. Bell and B. C. Clark helped to improve this Letter.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Rights and permissions
About this article
Cite this article
Vaniman, D., Bish, D., Chipera, S. et al. Magnesium sulphate salts and the history of water on Mars. Nature 431, 663–665 (2004). https://doi.org/10.1038/nature02973
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature02973
This article is cited by
-
A possible unique ecosystem in the endoglacial hypersaline brines in Antarctica
Scientific Reports (2023)
-
Softness of hydrated salt crystals under deliquescence
Nature Communications (2023)
-
Formation of manganese oxides on early Mars due to active halogen cycling
Nature Geoscience (2023)
-
Excellently balanced water-intercalation-type heat-storage oxide
Nature Communications (2022)
-
Anion-type modulates the effect of salt stress on saline lake bacteria
Extremophiles (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.