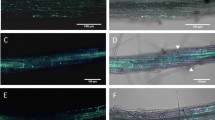
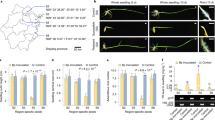
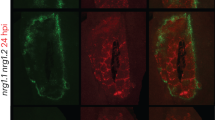
Abstract
Pathogens have evolved different strategies to overcome the various barriers that they encounter during infection of their hosts1. The rice blast fungus Magnaporthe grisea causes one of the most damaging diseases of cultivated rice and has emerged as a paradigm system for investigation of foliar pathogenicity. This fungus undergoes a series of well-defined developmental steps during leaf infection, including the formation of elaborate penetration structures (appressoria). This process has been studied in great detail2, and over thirty M. grisea genes that condition leaf infection have been identified3. Here we show a new facet of the M. grisea life cycle: this fungus can undergo a different (and previously uncharacterized) set of programmed developmental events that are typical of root-infecting pathogens. We also show that root colonization can lead to systemic invasion and the development of classical disease symptoms on the aerial parts of the plant. Gene-for-gene type specific disease resistance that is effective against rice blast in leaves also operates in roots. These findings have significant implications for fungal development, epidemiology, plant breeding and disease control.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Mendgen, K., Hahn, M. & Deising, H. Morphogenesis and mechanisms of penetration by plant pathogenic fungi. Annu. Rev. Phytopathol. 34, 367–386 (1996)
Tucker, S. L. & Talbot, N. J. Surface attachment and pre-penetration stage development by plant pathogenic fungi. Annu. Rev. Phytopathol. 39, 385–418 (2001)
Talbot, N. J. On the trail of a cereal killer: exploring the biology of Magnaporthe grisea. Annu. Rev. Microbiol. 57, 177–202 (2003)
International Rice Blast Genome Consortium. RICEBLAST.ORG, http://www.riceblast.org/ (2003).
Cannon, P. F. The newly recognized family Magnaporthaceae and its interrelationships. Systema Ascomycetum 13, 25–42 (1994)
Hornby, D. (ed.) Biology and Control of Take-all (Academic Press Inc., London, 1981)
Hornby, D. (ed.) Take-all Disease of Cereals: a Regional Perspective (CAB International, Wallingford, 1998)
Landschoot, P. J. & Jackson, N. Magnaporthe poae sp. nov., a hyphopodiate fungus with a Phialophora anamorph from grass roots in the United States. Mycol. Res. 93, 59–62 (1989)
Scott, D. B. & Deacon, J. W. Magnaporthe rhizophila sp. nov, a dark mycelial fungus with a Phialophora conidial state, from cereal roots in South-Africa. Trans. British Mycol. Soc. 81, 77–81 (1983)
Dufresne, M. & Osbourn, A. E. Definition of tissue-specific and general requirements for plant infection in a phytopathogenic fungus. Mol. Plant Microbe Interact. 14, 300–307 (2001)
De Jong, J. C., McCormack, B. J., Smirnoff, N. & Talbot, N. J. Glycerol generates turgor in rice blast. Nature 389, 244–245 (1997)
Howard, R. J. & Valent, B. Breaking and entering: host penetration by the fungal rice blast pathogen Magnaporthe grisea. Annu. Rev. Microbiol. 50, 491–512 (1996)
Rodrigues, F. A., Benhamou, N., Datnoff, L. E., Jones, J. B. & Belanger, R. R. Ultrastructural and cytochemical aspects of silicon-mediated rice blast resistance. Phytopathology 93, 535–546 (2003)
Talbot, N. J., Ebbole, D. J. & Hamer, J. E. Identification and characterization of MPG1, a gene involved in pathogenicity from the rice blast fungus Magnaporthe grisea. Plant Cell 5, 1575–1590 (1993)
Chumley, F. G. & Valent, B. Genetic analysis of melanin deficient, nonpathogenic mutants of Magnaporthe grisea. Mol. Plant Microbe Interact. 3, 135–143 (1990)
Valent, B., Farrall, L. & Chumley, F. Magnaporthe grisea genes for pathogenicity and virulence identified through a series of backcrosses. Genetics 127, 87–101 (1991)
Mitchell, T. K. & Dean, R. A. The cAMP-dependent protein kinase catalytic subunit is required for appressorium formation and pathogenesis by the rice blast pathogen Magnaporthe grisea. Plant Cell 7, 1869–1878 (1995)
Xu, J. R., Urban, M., Sweigard, J. A. & Hamer, J. E. The CPKA gene of Magnaporthe grisea is essential for appressorial penetration. Mol. Plant Microbe Interact. 10, 187–194 (1997)
Inoue, I., Namiki, F. & Tsuge, T. Plant colonization by the vascular wilt fungus Fusarium oxysporum requires FOW1, a gene encoding a mitochondrial protein. Plant Cell 14, 1869–1883 (2002)
Farman, M. L. et al. Analysis of the Structure of the AVR1–CO39 avirulence locus in virulent rice-infecting isolates of Magnaporthe grisea. Mol. Plant Microbe Interact. 15, 6–16 (2002)
Tian, D., Traw, M. B., Chen, J. Q., Kreitman, M. & Bergelson, J. Fitness costs of R-gene-mediated resistance in Arabidopsis thaliana. Nature 423, 74–76 (2003)
Ou, S. H. Rice Diseases (Commonwealth Mycological Institute, Kew, Surrey, UK, 1985)
Sosnowski, M., Ramsey, M., Murray, G., Scott, E. & Wilmshurst, C. Symptoms of blackleg (Leptosphaeria maculans) on the roots of canola in Australia. Plant Pathol. 50, 808 (2001)
Vereijssen, J., Schneider, H. J. M. & Termorshuizen, A. J. Possible root infection of Cercospora beticola in sugar beet. Eur. J. Plant Pathol. 110, 103–106 (2004)
Sambrook, J., Fritsch, E. F. & Maniatis, T. Molecular Cloning: a Laboratory Manual (Cold Spring Harbor Laboratory, Cold Spring Harbor, New York, 1989)
Lorang, J. M. et al. Green fluorescent protein is lighting up fungal biology. Appl. Environ. Microbiol. 67, 1987–1994 (2001)
Rho, H. S., Kang, S. & Lee, Y. H. Agrobacterium tumefaciens-mediated transformation of the plant pathogenic fungus, Magnaporthe grisea. Mol. Cells 12, 407–411 (2001)
Sweigard, J. A., Chumley, F., Carroll, A., Farrall, L. & Valent, B. A series of vectors for fungal transformation. Fungal Genet. Newslett. 44, 52–53 (1997)
Resendes, C. M., Geil, R. D. & Guinel, F. C. Mycorrhyzal development in a low nodulating pea mutant. New Phytol. 150, 563–572 (2001)
Gangopadhyay, S. & Row, K. V. K. Perennation of Pyricularia oryzae briosi et cav. in sclerotial state. Int. J. Trop. Plant Dis. 4, 187–192 (1986)
Acknowledgements
We thank the M. grisea community for providing fungal strains; L. Ciuffetti for the pCT74 reporter vector, and J. Deacon and R. Gutteridge for providing the images for Fig. 1l and 1m, respectively. A.S. has been supported by a Marie Curie fellowship from the European Community and by the Gatsby Charitable Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Rights and permissions
About this article
Cite this article
Sesma, A., Osbourn, A. The rice leaf blast pathogen undergoes developmental processes typical of root-infecting fungi. Nature 431, 582–586 (2004). https://doi.org/10.1038/nature02880
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature02880
This article is cited by
-
Upregulation of LmHxt1 gene is associated with reduced virulence of Leptosphaeria maculans on Brassica napus
Journal of Plant Pathology (2024)
-
Transcriptomic Profiling of Resistant and Susceptible Rice Cultivar Blast Resistance Genes During Magnaporthe oryzae Infection
Plant Molecular Biology Reporter (2023)
-
The Interaction between Rice Genotype and Magnaporthe oryzae Regulates the Assembly of Rice Root-Associated Microbiota
Rice (2021)
-
Imaging the invasion of rice roots by the bakanae agent Fusarium fujikuroi using a GFP-tagged isolate
European Journal of Plant Pathology (2021)
-
Rice Blast Lesions: an Unexplored Phyllosphere Microhabitat for Novel Antagonistic Bacterial Species Against Magnaporthe oryzae
Microbial Ecology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.