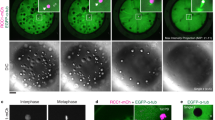
Abstract
Polarity establishment requires a symmetry-breaking event, resulting in an axis along which determinants are segregated. In Caenorhabditis elegans, oocytes are apolar and are triggered to polarize rapidly along one axis after fertilization. The establishment of this first polarity axis is revealed by the asymmetric distribution of PAR proteins and cortical activity in the one-celled embryo. Current evidence suggests that the centrosome–pronucleus complex contributed by the sperm is involved in defining the polarization axis1,2,3,4,5,6. Here we directly assess the contribution of the centrosome to polarity establishment by laser ablating the centrosome before and during polarization. We find that the centrosome is required to initiate polarity but not to maintain it. Initiation of polarity coincides with the proximity of the centrosome to the cortex and the assembly of pericentriolar material on the immature sperm centrosome. Depletion of microtubules or the microtubule nucleator γ-tubulin did not affect polarity establishment. These results demonstrate that the centrosome provides an initiating signal that polarizes C. elegans embryos and indicate that this signalling event might be independent of the role of the centrosome as a microtubule nucleator.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hamill, D. R., Severson, A. F., Carter, J. C. & Bowerman, B. Centrosome maturation and mitotic spindle assembly in C. elegans require SPD-5, a protein with multiple coiled-coil domains. Dev. Cell 3, 673–684 (2002)
O'Connell, K. F., Maxwell, K. N. & White, J. G. The spd-2 gene is required for polarization of the anteroposterior axis and formation of the sperm asters in the Caenorhabditis elegans zygote. Dev. Biol. 222, 55–70 (2000)
Goldstein, B. & Hird, S. N. Specification of the anteroposterior axis in Caenorhabditis elegans. Development 122, 1467–1474 (1996)
Sadler, P. L. & Shakes, D. C. Anucleate Caenorhabditis elegans sperm can crawl, fertilize oocytes and direct anterior–posterior polarization of the 1-cell embryo. Development 127, 355–366 (2000)
Cuenca, A. A., Schetter, A., Aceto, D., Kemphues, K. & Seydoux, G. Polarization of the C. elegans zygote proceeds via distinct establishment and maintenance phases. Development 130, 1255–1265 (2003)
Wallenfang, M. R. & Seydoux, G. Polarization of the anterior–posterior axis of C. elegans is a microtubule-directed process. Nature 408, 89–92 (2000)
Boyd, L., Guo, S., Levitan, D., Stinchcomb, D. T. & Kemphues, K. J. PAR-2 is asymmetrically distributed and promotes association of P granules and PAR-1 with the cortex in C. elegans embryos. Development 122, 3075–3084 (1996)
Hung, T. J. & Kemphues, K. J. PAR-6 is a conserved PDZ domain-containing protein that colocalizes with PAR-3 in Caenorhabditis elegans embryos. Development 126, 127–135 (1999)
Etemad-Moghadam, B., Guo, S. & Kemphues, K. J. Asymmetrically distributed PAR-3 protein contributes to cell polarity and spindle alignment in early C. elegans embryos. Cell 83, 743–752 (1995)
Guo, S. & Kemphues, K. J. par-1, a gene required for establishing polarity in C. elegans embryos, encodes a putative Ser/Thr kinase that is asymmetrically distributed. Cell 81, 611–620 (1995)
Pelletier, L. et al. The Caenorhabditis elegans centrosomal protein SPD-2 is required for both pericentriolar material recruitment and centriole duplication. Curr. Biol. 14, 863–873 (2004)
Hannak, E. et al. The kinetically dominant assembly pathway for centrosomal asters in Caenorhabditis elegans is gamma-tubulin dependent. J. Cell Biol. 157, 591–602 (2002)
Albertson, D. G. Formation of the first cleavage spindle in nematode embryos. Dev. Biol. 101, 61–72 (1984)
Strome, S. et al. Spindle dynamics and the role of gamma-tubulin in early Caenorhabditis elegans embryos. Mol. Biol. Cell 12, 1751–1764 (2001)
Kemp, C. A., Kopish, K. R., Zipperlen, P., Ahringer, J. & O'Connell, K. F. Centrosome maturation and duplication in C. elegans require the coiled-coil protein SPD-2. Dev. Cell 6, 511–523 (2004)
Stevenson, V. A., Kramer, J., Kuhn, J. & Theurkauf, W. E. Centrosomes and the Scrambled protein coordinate microtubule-independent actin reorganization. Nature Cell Biol. 3, 68–75 (2001)
Berdnik, D. & Knoblich, J. A. Drosophila Aurora-A is required for centrosome maturation and actin-dependent asymmetric protein localization during mitosis. Curr. Biol. 12, 640–647 (2002)
Piel, M., Nordberg, J., Euteneuer, U. & Bornens, M. Centrosome-dependent exit of cytokinesis in animal cells. Science 291, 1550–1553 (2001)
Gonczy, P. et al. Dissection of cell division processes in the one cell stage Caenorhabditis elegans embryo by mutational analysis. J. Cell Biol. 144, 927–946 (1999)
Oegema, K., Desai, A., Rybina, S., Kirkham, M. & Hyman, A. A. Functional analysis of kinetochore assembly in Caenorhabditis elegans. J. Cell Biol. 153, 1209–1226 (2001)
Praitis, V., Casey, E., Collar, D. & Austin, J. Creation of low-copy integrated transgenic lines in Caenorhabditis elegans. Genetics 157, 1217–1226 (2001)
Kirkham, M., Muller-Reichert, T., Oegema, K., Grill, S. & Hyman, A. A. SAS-4 is a C. elegans centriolar protein that controls centrosome size. Cell 112, 575–587 (2003)
Grill, S. W., Howard, J., Schaffer, E., Stelzer, E. H. & Hyman, A. A. The distribution of active force generators controls mitotic spindle position. Science 301, 518–521 (2003)
Pichler, S. et al. OOC-3, a novel putative transmembrane protein required for establishment of cortical domains and spindle orientation in the P(1) blastomere of C. elegans embryos. Development 127, 2063–2073 (2000)
Acknowledgements
We thank: A. Desai, S. Eaton, C. Hoege, K. Oegema, N. Özlü, L. Pelletier, S. Quintin, A. Schlaitz, S. Schonegg, M. Srayko, J. Stear, A. Tudor-Constantinescu and M. van Breugel for comments on the manuscript; S. Grill for advice on laser ablation; A. Pozniakovsky for modified GFP vectors; and G. Seydoux for the gift of JH1380 worms. Some of the worm strains used in this study were obtained from the Caenorhabditis Genetics Center, funded by the NIH.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Supplementary information
Supplementary Figure 1
Effect of PCM depletion on contractile polarity establishment. (PDF 2199 kb)
Supplementary Figure 2
Analysis of centrosome-cortex juxtaposition in embryos depleted of g-tubulin, SPD-2, and SPD-5. (PDF 1794 kb)
Supplementary Figure 3
Centrosomal microtubules at the time of polarity initiation in control, spd-2(RNAi), and γ-tubulin(RNAi) embryos. (PDF 1956 kb)
Supplementary Figure 4
PAR-2 and ruffling polarity analysis following microtubule depletion by β-tubulin(RNAi) + 15 μm nocodazole. (PDF 1364 kb)
Supplementary Movie 1
Centrosomes are adjacent to the cortex at the time of polarity initiation. Time lapse movie of a GFP::PAR-2; GFP::SPD-2 embryo prior to and during polarity establishment. The movie plays at roughly 120X actual speed. Embryo posterior is to the right. (MP4 694 kb)
Supplementary Movie 2
Centrosome ablation prevents polarity establishment. The GFP::SPD-2-labelled centrosome (indicated by the red circle) was ablated (red "x"); GFP::PAR-2 and cortical ruffling were used to assess polarity. The movie plays at roughly 120X actual speed. The embryo meiotic pole is to the left. (MP4 911 kb)
Supplementary Movie 3
Centrosome ablation prevents polarity establishment. The GFP::SPD-2-labelled centrosome (indicated by the red circle) was ablated (red "x"); GFP::PAR-2 was monitored to assess polarity. A failure in polar body extrusion is evident following centrosome ablation, although this defect was also observed occaisionally in control ablation embryos. The movie plays at roughly 120X actual speed. The embryo meiotic pole is to the left. (MP4 607 kb)
Supplementary Movie 4
Control ablation does not affect polarity establishment. An area of cytoplasm (indicated by the red circle) in the vicinity of the GFP::SPD-2-labelled centrosome was irradiated (red "x"), as for centrosome ablations. GFP::PAR-2 was used to assess polarity. The centrosomal SPD-2 is photobleached by the ablation but returns several frames later. The movie plays at roughly 120X actual speed. Embryo posterior is to the right. (MP4 547 kb)
Supplementary Movie 5
Centrosome ablation after polarity initiation does not affect the propagation of posterior polarity. The GFP::SPD-2-labelled centrosomes (indicated by the red circle) were ablated (red "x") during the expansion of the posterior domain (boundary indicated by green arrows). GFP::PAR-2 was used to monitor polarity pre- and post-ablation. The movie plays at roughly 120X actual speed. Embryo posterior is to the right. (MP4 664 kb)
Supplementary Movie 6
Centrosome ablation after polarity establishment does not affect the maintenance of posterior polarity. The GFP::SPD-2-labelled centrosomes (indicated by the red circles) were ablated (red "x") after the posterior domain had been formed (PAR-2 occupied half the embryo cortex). GFP::PAR-2 was used to monitor polarity pre- and post-ablation. The movie plays at roughly 120X actual speed. Embryo posterior is to the right. (MP4 724 kb)
Supplementary Movie 7
Cortical control ablation during polarity initiation does not affect the extension of posterior polarity. A small region of the cortex (indicated by the red circle) was ablated (red "x") during the formation of the posterior domain. GFP::PAR-2 was used to monitor polarity pre- and post-ablation; GFP::SPD-2 marks the centrosomes. Cortical damage, confirming the effectiveness of the ablation, can be seen later in the recording (indicated by a green arrow) The movie plays at roughly 120X actual speed. Embryo posterior is to the right. (MP4 735 kb)
Rights and permissions
About this article
Cite this article
Cowan, C., Hyman, A. Centrosomes direct cell polarity independently of microtubule assembly in C. elegans embryos. Nature 431, 92–96 (2004). https://doi.org/10.1038/nature02825
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature02825
This article is cited by
-
Geometric cues stabilise long-axis polarisation of PAR protein patterns in C. elegans
Nature Communications (2020)
-
Guiding self-organized pattern formation in cell polarity establishment
Nature Physics (2019)
-
The role of polarisation of circulating tumour cells in cancer metastasis
Cellular and Molecular Life Sciences (2019)
-
Establishment of the PAR-1 cortical gradient by the aPKC-PRBH circuit
Nature Chemical Biology (2018)
-
Differences in the genetic control of early egg development and reproduction between C. elegans and its parthenogenetic relative D. coronatus
EvoDevo (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.