Abstract
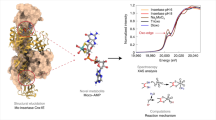
The molybdenum cofactor is part of the active site of all molybdenum-dependent enzymes1, except nitrogenase. The molybdenum cofactor consists of molybdopterin, a phosphorylated pyranopterin2, with an ene-dithiolate coordinating molybdenum. The same pyranopterin-based cofactor is involved in metal coordination of the homologous tungsten-containing enzymes found in archea3. The molybdenum cofactor is synthesized by a highly conserved biosynthetic pathway4. In plants, the multidomain protein Cnx1 catalyses the insertion of molybdenum into molybdopterin. The Cnx1 G domain (Cnx1G), whose crystal structure has been determined in its apo form, binds molybdopterin with high affinity and participates in the catalysis of molybdenum insertion. Here we present two high-resolution crystal structures of Cnx1G in complex with molybdopterin and with adenylated molybdopterin (molybdopterin–AMP), a mechanistically important intermediate. Molybdopterin–AMP is the reaction product of Cnx1G and is subsequently processed in a magnesium-dependent reaction by the amino-terminal E domain of Cnx1 to yield active molybdenum cofactor. The unexpected identification of copper bound to the molybdopterin dithiolate sulphurs in both structures, coupled with the observed copper inhibition of Cnx1G activity, provides a molecular link between molybdenum and copper metabolism.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hille, R. The mononuclear molybdenum enzymes. Chem. Rev. 96, 2757–2816 (1996)
Romao, M. J. et al. Crystal structure of the xanthine oxidase-related aldehyde oxido- reductase from D. gigas. Science 270, 1170–1176 (1995)
Chan, M. K., Mukund, S., Kletzin, A., Adams, M. W. W. & Rees, D. C. Structure of a hyperthermophilic tungstopterin, aldehyde ferredoxin oxidoreductase. Science 267, 1463–1469 (1995)
Mendel, R. R. & Schwarz, G. Biosynthesis and molecular biology of the molybdenum cofactor (Moco). Met. Ions Biol. Syst. 39, 317–368 (2002)
Stiefel, E. I. Molybdenum bolsters the bioinorganic brigade. Science 272, 1599–1600 (1996)
Enemark, J. H. & Cosper, M. M. Molybdenum enzymes and sulfur metabolism. Met. Ions Biol. Syst. 39, 621–654 (2002)
Mendel, R. R. & Schwarz, G. Molybdoenzymes and molybdenum cofactor in plants. Crit. Rev. Plant. Sci. 18, 33–69 (1999)
Johnson, J. L. et al. Inborn errors of molybdenum metabolism: combined deficiencies of sulfite oxidase and xanthine dehydrogenase in a patient lacking the molybdenum cofactor. Proc. Natl Acad. Sci. USA 77, 3715–3719 (1980)
Shih, V. E. et al. Sulfite oxidase deficiency. Biochemical and clinical investigations of a hereditary metabolic disorder in sulfur metabolism. N. Engl. J. Med. 297, 1022–1028 (1977)
Kuper, J., Winking, J., Hecht, H. J., Mendel, R. R. & Schwarz, G. The active site of the molybdenum cofactor biosynthetic protein domain Cnx1G. Arch. Biochem. Biophys. 411, 36–46 (2003)
Schwarz, G. et al. The molybdenum cofactor biosynthetic protein Cnx1 complements molybdate-repairable mutants, transfers molybdenum to the metal binding pterin, and is associated with the cytoskeleton. Plant Cell 12, 2455–2472 (2000)
Kuper, J. et al. In vivo detection of molybdate-binding proteins using a competition assay with ModE in Escherichia coli. FEMS Microbiol. Lett. 218, 187–193 (2003)
Kisker, C. et al. Molecular basis of sulfite oxidase deficiency from the structure of sulfite oxidase. Cell 91, 973–983 (1997)
Kuper, J., Palmer, T., Mendel, R. R. & Schwarz, G. Mutations in the molybdenum cofactor biosynthetic protein Cnx1G from Arabidopsis thaliana define functions for molybdopterin bind, Mo-insertion and molybdenum cofactor stabilization. Proc. Natl Acad. Sci. USA 97, 6475–6480 (2000)
Lake, M. W., Wuebbens, M. M., Rajagopalan, K. V. & Schindelin, H. Mechanism of ubiquitin activation revealed by the structure of a bacterial MoeB–MoaD complex. Nature 414, 325–329 (2001)
Schindelin, H., Kisker, C., Hilton, J., Rajagopalan, K. V. & Rees, D. C. Crystal structure of DMSO reductase: redox-linked changes in molybdopterin coordination. Science 272, 1615–1621 (1996)
Schrader, N. et al. The crystal structure of plant sulfite oxidase provides insights into sulfite oxidation in plants and animals. Structure 11, 1251–1263 (2003)
Santamaria-Araujo, J. A. et al. The tetrahydropyranopterin structure of the sulfur-free and metal-free molybdenum cofactor precursor. J. Biol. Chem. 279, 15994–15999 (2004)
Bertero, M. G. et al. Insights into the respiratory electron transfer pathway from the structure of nitrate reductase A. Nature Struct. Biol. 10, 681–687 (2003)
Nason, A. et al. In vitro formation of assimilatory reduced nicotinamide adenine dinucleotide phosphate: nitrate reductase from a Neurospora mutant and a component of molybdenum-enzymes. Proc. Natl Acad. Sci. USA 68, 3242–3246 (1971)
Xiang, S., Nichols, J., Rajagopalan, K. V. & Schindelin, H. The crystal structure of Escherichia coli MoeA and its relationship to the multifunctional protein Gephyrin. Structure 9, 299–310 (2001)
Schrag, J. D. et al. The crystal structure of Escherichia coli MoeA, a protein from the molybdopterin synthesis pathway. J. Mol. Biol. 310, 419–431 (2001)
Moorhead, G. B. et al. Purification of a plant nucleotide pyrophosphatase as a protein that interferes with nitrate reductase and glutamine synthetase assays. Eur. J. Biochem. 270, 1356–1362 (2003)
Mercer, J. F. The molecular basis of copper-transport diseases. Trends Mol. Med. 7, 64–69 (2001)
Mason, J. Thiomolybdates: mediators of molybdenum toxicity and enzyme inhibitors. Toxicology 42, 99–109 (1986)
Navaza, J. AMORE—an automated package for molecular replacement. Acta Crystallogr. A 50, 157–163 (1994)
Jones, T. A., Zou, J. Y., Cowan, S. W. & Kjeldgaard, M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr. A 47, 110–119 (1991)
Murshudov, G., Vagin, A. & Dodson, E. Refinement of macromolecular structures by the maximum likelihood method. Acta Crystallogr. D 53, 240–255 (1997)
Guse, A. et al. Biochemical and structural analysis of the molybdenum cofactor biosynthesis protein MobA. J. Biol. Chem. 278, 25302–25307 (2003)
Nicholls, A., Sharp, K. A. & Honig, B. Protein folding and association: insights from the interfacial and thermodynamic properties of hydrocarbons. Proteins Struct. Funct. Genet. 11, 281–296 (1991)
Acknowledgements
We thank R. N. Pau for inspiring discussion; T. Otte and F. Koenig for technical assistance; the staff at beamlines BW6 at DESY and PSF-BL2 at BESSY; and V. Wray for critically reading the manuscript. This work was supported by grants from the Deutsche Forschungsgemeinschaft (to H.J.H., R.R.M. and G.S.), and the Fonds der Chemischen Industrie and the Fritz Thyssen Stiftung (to R.R.M.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Supplementary information
Supplementary Figure 1
Coordination of Cu-MPT and Cu-MPT-AMP. (DOC 196 kb)
Supplementary Figure 2
Structural comparison between Cnx1G and S583A. (DOC 867 kb)
Supplementary Figure 3
Structural comparison of MPT-AMP. (DOC 210 kb)
Supplementary Figure 4
Structural comparison between S583A and the Cnx1E-homologous MoeA. (DOC 1155 kb)
Supplementary Table 1
Data collection and refinement statistics. (DOC 32 kb)
Rights and permissions
About this article
Cite this article
Kuper, J., Llamas, A., Hecht, HJ. et al. Structure of the molybdopterin-bound Cnx1G domain links molybdenum and copper metabolism. Nature 430, 803–806 (2004). https://doi.org/10.1038/nature02681
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature02681
This article is cited by
-
Mechanism of molybdate insertion into pterin-based molybdenum cofactors
Nature Chemistry (2021)
-
Identification of miRNA-mediated drought responsive multi-tiered regulatory network in drought tolerant rice, Nagina 22
Scientific Reports (2017)
-
The biosynthesis of the molybdenum cofactors
JBIC Journal of Biological Inorganic Chemistry (2015)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.