Abstract
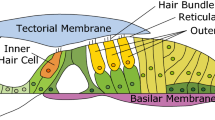
Sensory receptor cells of the mammalian cochlea are morphologically and functionally dichotomized. Inner hair cells transmit auditory information to the brain, whereas outer hair cells (OHC) amplify the mechanical signal, which is then transduced by inner hair cells1. Amplification by OHCs is probably mediated by their somatic motility2,3 in a mechanical feedback process. OHC motility in vivo is thought to be driven by the cell's receptor potential. The first steps towards the generation of the receptor potential are the deflection of the stereociliary bundle4, and the subsequent flow of transducer current through the mechanosensitive transducer channels located at their tips5. Quantitative relations between transducer currents and basilar membrane displacements are lacking, as well as their variation along the cochlear length. To address this, we simultaneously recorded OHC transducer currents (or receptor potentials) and basilar membrane motion in an excised and bisected cochlea, the hemicochlea6. This preparation permits recordings from adult OHCs at various cochlear locations while the basilar membrane is mechanically stimulated. Furthermore, the stereocilia are deflected by the same means of stimulation as in vivo. Here we show that asymmetrical transducer currents and receptor potentials are significantly larger than previously thought, they possess a highly restricted dynamic range and strongly depend on cochlear location.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Dallos, P. The active cochlea. J. Neurosci. 12, 4575–4585 (1992)
Brownell, W. E., Bader, C. R., Bertrand, D. & de Ribaupierre, Y. Evoked mechanical responses in isolated cochlear outer hair cells. Science 227, 194–196 (1985)
Liberman, M. C. et al. Prestin is required for outer hair cell electromotility and the cochlear amplifier. Nature 419, 300–304 (2002)
Hudspeth, A. J. & Corey, D. P. Sensitivity, polarity and conductance change in the response of vertebrate hair cells to controlled mechanical stimuli. Proc. Natl Acad. Sci. USA 74, 2407–2411 (1977)
Denk, W., Holt, J. R., Shepherd, G. M. & Corey, D. P. Calcium imaging of single stereocilia in hair cells: localization of transduction channels at both ends of tip links. Neuron 15, 1311–1321 (1995)
Richter, C. P., Evans, B. N., Edge, R. & Dallos, P. Basilar membrane vibration in the gerbil hemicochlea. J. Neurophysiol. 79, 2255–2264 (1998)
Ashmore, J. F., Kolston, P. J. & Mammano, F. in Biophysics of Hair Cell Sensory Systems (eds Duifhuis, H., Horst, J. W., Dijk, P. & van Netten, S. M.) 151–158 (World Scientific, Singapore, 1993)
Kros, C. J., Rüsch, A. & Richardson, G. P. Mechano-electrical transducer currents in hair cells of the cultured neonatal mouse cochlea. Proc. R. Soc. Lond. B 249, 185–193 (1992)
Kennedy, H. J., Evans, M. G., Crawford, A. C. & Fettiplace, R. Fast adaptation of mechanoelectrical transducer channels in mammalian cochlear hair cells. Nature Neurosci. 6, 832–836 (2003)
Müller, M. The cochlear place-frequency map of the adult and developing Mongolian gerbil. Hear. Res. 94, 148–156 (1996)
Ricci, A. J., Crawford, A. C. & Fettiplace, R. Tonotopic variation in the conductance of the hair cell mechanotransducer channel. Neuron 40, 983–990 (2003)
Russell, I. J. & Sellick, P. M. Low-frequency characteristics of intracellularly recorded receptor potentials in guinea-pig cochlear hair cells. J. Physiol. (Lond.) 338, 179–206 (1983)
Russell, I. J., Cody, A. R. & Richardson, G. P. The responses of inner and outer hair cells in the basal turn of the guinea-pig cochlea and in the mouse cochlea grown in vitro. Hear. Res. 22, 199–216 (1986)
Dallos, P. Response characteristics of mammalian cochlear hair cells. J. Neurosci. 5, 1591–1608 (1985)
Dallos, P. Organ of Corti kinematics. J. Assoc. Res. Otolaryngol. 4, 416–421 (2003)
Géléoc, G. S. et al. A quantitative comparison of mechanoelectrical transduction in vestibular and auditory hair cells of neonatal mice. Proc. R. Soc. Lond. B 264, 611–621 (1997)
Cheatham, M. A. et al. Assessment of hair-cell transducer function in prestin knockout mice. Assoc. Res. Otolaryngol. Abstr. 1248 (2004)
Gale, J. E. & Ashmore, J. F. Charge displacement induced by rapid stretch in the basolateral membrane of the guinea-pig outer hair cell. Proc. R. Soc. Lond. B 255, 243–249 (1994)
Zheng, J. et al. Prestin is the motor protein of cochlear outer hair cells. Nature 405, 149–155 (2000)
Corey, D. P. & Hudspeth, A. J. Ionic basis of the receptor potential in a vertebrate hair cell. Nature 281, 675–677 (1979)
Oliver, D. et al. Intracellular anions as the voltage sensor of prestin, the outer hair cell motor protein. Science 292, 2340–2343 (2001)
Crawford, A. C., Evans, M. G. & Fettiplace, R. The actions of calcium on the mechano-electrical transducer current of turtle hair cells. J. Physiol. (Lond.) 434, 369–398 (1991)
Dallos, P., Santos-Sacchi, J. & Flock, Å. Intracellular recordings from cochlear outer hair cells. Science 218, 582–584 (1982)
Russell, I. J., Richardson, G. P. & Cody, A. R. Mechanosensitivity of mammalian auditory hair cells in vitro. Nature 321, 517–519 (1986)
Housley, G. D. & Ashmore, J. F. Ionic currents of outer hair cells isolated from the guinea-pig cochlea. J. Physiol. (Lond.) 448, 73–98 (1992)
Crawford, A. C. & Fettiplace, R. An electrical tuning mechanism in turtle cochlear hair cells. J. Physiol. (Lond.) 312, 377–412 (1981)
Holt, J. R. et al. A chemical-genetic strategy implicates myosin-1c in adaptation by hair cells. Cell 108, 371–381 (2002)
Mammano, F. & Ashmore, J. F. Differential expression of outer hair cell potassium currents in the isolated cochlea of the guinea-pig. J. Physiol. (Lond.) 496, 639–646 (1996)
Dallos, P. Neurobiology of cochlear inner and outer hair cells: intracellular recordings. Hear. Res. 22, 185–198 (1986)
Cooper, N. P. & Rhode, W. S. Mechanical responses to two-tone distortion products in the apical and basal turns of the mammalian cochlea. J. Neurophysiol. 78, 261–270 (1997)
He, D. Z. Z., Jia, S. P. & Dallos, P. Prestin and the dynamic stiffness of cochlear outer hair cells. J. Neurosci. 23, 9089–9096 (2003)
Acknowledgements
This work has been supported by National Institute of Health grants to D.Z.Z.H., and to P.D.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Rights and permissions
About this article
Cite this article
He, D., Jia, S. & Dallos, P. Mechanoelectrical transduction of adult outer hair cells studied in a gerbil hemicochlea. Nature 429, 766–770 (2004). https://doi.org/10.1038/nature02591
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature02591
This article is cited by
-
The sensitivity of mechanoelectrical transduction response phase to acoustic overstimulation is calcium-dependent
Pflügers Archiv - European Journal of Physiology (2024)
-
The summating potential polarity encodes the ear health condition
Cellular and Molecular Life Sciences (2023)
-
The Long Outer-Hair-Cell RC Time Constant: A Feature, Not a Bug, of the Mammalian Cochlea
Journal of the Association for Research in Otolaryngology (2023)
-
The origin of mechanical harmonic distortion within the organ of Corti in living gerbil cochleae
Communications Biology (2021)
-
Spectral Ripples in Round-Window Cochlear Microphonics: Evidence for Multiple Generation Mechanisms
Journal of the Association for Research in Otolaryngology (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.