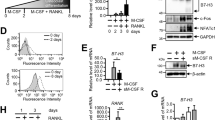
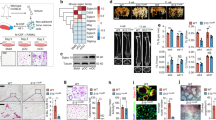
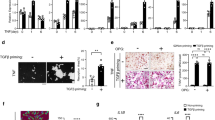
Abstract
Costimulatory signals are required for activation of immune cells1, but it is not known whether they contribute to other biological systems. The development and homeostasis of the skeletal system depend on the balance between bone formation and resorption2,3. Receptor activator of NF-κB ligand (RANKL) regulates the differentiation of bone-resorbing cells, osteoclasts, in the presence of macrophage-colony stimulating factor (M-CSF)4,5. But it remains unclear how RANKL activates the calcium signals that lead to induction of nuclear factor of activated T cells c1, a key transcription factor for osteoclastogenesis6. Here we show that mice lacking immunoreceptor tyrosine-based activation motif (ITAM)7-harbouring adaptors8,9,10, Fc receptor common γ subunit (FcRγ) and DNAX-activating protein (DAP)12, exhibit severe osteopetrosis owing to impaired osteoclast differentiation. In osteoclast precursor cells, FcRγ and DAP12 associate with multiple immunoreceptors11,12,13,14,15 and activate calcium signalling through phospholipase Cγ. Thus, ITAM-dependent costimulatory signals activated by multiple immunoreceptors are essential for the maintenance of bone homeostasis. These results reveal that RANKL and M-CSF are not sufficient to activate the signals required for osteoclastogenesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Lenschow, D. J., Walunas, T. L. & Bluestone, J. A. CD28/B7 system of T cell costimulation. Annu. Rev. Immunol. 14, 233–258 (1996)
Karsenty, G. & Wagner, E. F. Reaching a genetic and molecular understanding of skeletal development. Dev. Cell. 2, 389–406 (2002)
Rodan, G. A. & Martin, T. J. Therapeutic approaches to bone diseases. Science 289, 1508–1514 (2000)
Teitelbaum, S. L. & Ross, F. P. Genetic regulation of osteoclast development and function. Nature Rev. Genet. 4, 638–649 (2003)
Boyle, W. J., Simonet, W. S. & Lacey, D. L. Osteoclast differentiation and activation. Nature 423, 337–342 (2003)
Takayanagi, H. et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev. Cell. 3, 889–901 (2002)
Reth, M. Antigen receptor tail clue. Nature 338, 383–384 (1989)
Perez-Montfort, R., Kinet, J. P. & Metzger, H. A previously unrecognized subunit of the receptor for immunoglobulin E. Biochemistry 22, 5722–5728 (1983)
Olcese, L. et al. Human killer cell activatory receptors for MHC class I molecules are included in a multimeric complex expressed by natural killer cells. J. Immunol. 158, 5083–5086 (1997)
Lanier, L. L., Corliss, B. C., Wu, J., Leong, C. & Phillips, J. H. Immunoreceptor DAP12 bearing a tyrosine-based activation motif is involved in activating NK cells. Nature 391, 703–707 (1998)
Kim, N., Takami, M., Rho, J., Josien, R. & Choi, Y. A novel member of the leukocyte receptor complex regulates osteoclast differentiation. J. Exp. Med. 195, 201–209 (2002)
Kubagawa, H., Burrows, P. D. & Cooper, M. D. A novel pair of immunoglobulin-like receptors expressed by B cells and myeloid cells. Proc. Natl Acad. Sci. USA 94, 5261–5266 (1997)
Colonna, M. TREMs in the immune system and beyond. Nature Rev. Immunol. 3, 445–453 (2003)
Dietrich, J., Cella, M., Seiffert, M., Buhring, H. J. & Colonna, M. Cutting edge: signal-regulatory protein β1 is a DAP12-associated activating receptor expressed in myeloid cells. J. Immunol. 164, 9–12 (2000)
Tomasello, E. et al. Association of signal-regulatory proteins β with KARAP/DAP-12. Eur. J. Immunol. 30, 2147–2156 (2000)
Kaifu, T. et al. Osteopetrosis and thalamic hypomyelinosis with synaptic degeneration in DAP12-deficient mice. J. Clin. Invest. 111, 323–332 (2003)
Takayanagi, H. et al. T-cell-mediated regulation of osteoclastogenesis by signalling cross-talk between RANKL and IFN-γ. Nature 408, 600–605 (2000)
Tomasello, E. et al. Gene structure, expression pattern, and biological activity of mouse killer cell activating receptor-associated protein (KARAP)/DAP-12. J. Biol. Chem. 273, 34115–34119 (1998)
Takahashi, N. et al. Osteoblastic cells are involved in osteoclast formation. Endocrinology 123, 2600–2602 (1988)
Takai, T., Li, M., Sylvestre, D., Clynes, R. & Ravetch, J. V. FcR γ chain deletion results in pleiotrophic effector cell defects. Cell 76, 519–529 (1994)
Takai, T. Roles of Fc receptors in autoimmunity. Nature Rev. Immunol. 2, 580–592 (2002)
Cerwenka, A. & Lanier, L. L. Natural killer cells, viruses and cancer. Nature Rev. Immunol. 1, 41–49 (2001)
Aoki, K. et al. The tyrosine phosphatase SHP-1 is a negative regulator of osteoclastogenesis and osteoclast resorbing activity: increased resorption and osteopenia in mev/mev mutant mice. Bone 25, 261–267 (1999)
Takeshita, S. et al. SHIP-deficient mice are severely osteoporotic due to increased numbers of hyper-resorptive osteoclasts. Nature Med. 8, 943–949 (2002)
Paloneva, J. et al. Mutations in two genes encoding different subunits of a receptor signaling complex result in an identical disease phenotype. Am. J. Hum. Genet. 71, 656–662 (2002)
Paloneva, J. et al. DAP12/TREM2 deficiency results in impaired osteoclast differentiation and osteoporotic features. J. Exp. Med. 198, 669–675 (2003)
Cella, M. et al. Impaired differentiation of osteoclasts in TREM-2-deficient individuals. J. Exp. Med. 198, 645–651 (2003)
Ujike, A. et al. Modulation of immunoglobulin (Ig)E-mediated systemic anaphylaxis by low-affinity Fc receptors for IgG. J. Exp. Med. 189, 1573–1579 (1999)
Takayanagi, H. et al. RANKL maintains bone homeostasis through c-Fos-dependent induction of interferon-β. Nature 416, 744–749 (2002)
Komarova, S. V., Pilkington, M. F., Weidema, A. F., Dixon, S. J. & Sims, S. M. RANK ligand-induced elevation of cytosolic Ca2+ accelerates nuclear translocation of nuclear factor κB in osteoclasts. J. Biol. Chem. 278, 8286–8293 (2003)
Acknowledgements
We thank J. V. Ravetch, H. Kubagawa and M. D. Cooper for providing materials, and M. Kaji, A. Sugahara, Y. Ito, K. Maya, A. Sato, A. Nakamura, M. Isobe, T. Yokochi, A. Izumi, T. Kohro, Y. Matsui, H. Murayama, K. Sato, M. Asagiri and I. Kawai for technical assistance and discussion. This work was supported in part by a grant for Advanced Research on Cancer from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, the CREST and PRESTO programs of Japan Science and Technology Agency (JST), grants for the 21st century COE program ‘Frontier Research on Molecular Destruction and Reconstruction of Tooth and Bone’ and ‘Center for Innovative Therapeutic Development Towards the Conquest of Signal Transduction Diseases’, Grants-in-Aid for Scientific Research from JSPS and MEXT, Health Sciences Research Grants from the Ministry of Health, Labour and Welfare of Japan, grants of the Virtual Research Institute of Aging of Nippon Boehringer Ingelheim, Mochida Medical and Pharmaceutical Research Foundation and a grant from Japan Orthopaedics and Traumatology Foundation.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Supplementary information
Supplementary Figures
Supplementary figure 1: Analysis of the rescue effects by osteoblast and the involvement of FcRγ; Supplementary figure 2: Bone morphometric and histological analysis of DAP12-/-FcRg-/- (DKO) mice; Supplementary figure 3: ITAM-harbouring adaptor-associated immunoreceptors in osteoclast lineage cells; Supplementary figure 4: Intracellular signalling events downstream of ITAM-harbouring adaptors. (PDF 328 kb)
Rights and permissions
About this article
Cite this article
Koga, T., Inui, M., Inoue, K. et al. Costimulatory signals mediated by the ITAM motif cooperate with RANKL for bone homeostasis. Nature 428, 758–763 (2004). https://doi.org/10.1038/nature02444
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature02444
This article is cited by
-
Recent advances of NFATc1 in rheumatoid arthritis-related bone destruction: mechanisms and potential therapeutic targets
Molecular Medicine (2024)
-
The role of TRPV2 as a regulator on the osteoclast differentiation during orthodontic tooth movement in rats
Scientific Reports (2023)
-
Osteoclast differentiation and dynamic mRNA expression during mice embryonic palatal bone development
Scientific Reports (2023)
-
Artemether Inhibits RANKL-Induced Osteoclast Differentiation and Prevents Bone Loss in Ovariectomized Mice
Revista Brasileira de Farmacognosia (2023)
-
Thioacetamide promotes osteoclast transformation of bone marrow macrophages by influencing PI3K/AKT pathways
Journal of Orthopaedic Surgery and Research (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.