Abstract
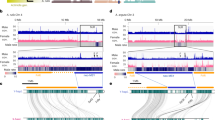
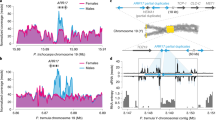
Many diverse systems for sex determination have evolved in plants and animals1,2,3. One involves physically distinct (heteromorphic) sex chromosomes (X and Y, or Z and W) that are homozygous in one sex (usually female) and heterozygous in the other (usually male). Sex chromosome evolution is thought to involve suppression of recombination around the sex determination genes, rendering permanently heterozygous a chromosomal region that may then accumulate deleterious recessive mutations by Muller's ratchet, and fix deleterious mutations by hitchhiking as nearby favourable mutations are selected on the Y chromosome4,5. Over time, these processes may cause the Y chromosome to degenerate and to diverge from the X chromosome over much of its length; for example, only 5% of the human Y chromosome still shows X–Y recombination6. Here we show that papaya contains a primitive Y chromosome, with a male-specific region that accounts for only about 10% of the chromosome but has undergone severe recombination suppression and DNA sequence degeneration. This finding provides direct evidence for the origin of sex chromosomes from autosomes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Westergaard, M. The mechanism of sex determination in flowering plants. Adv. Genet. 9, 217–281 (1958)
White, M. J. D. in Insect Reproduction (eds Leather, S. R. & Hardie, J.) 57–94 (Cambridge Univ. Press, Cambridge, 1973)
Ohno, S. Sex Chromosome and Sex-linked Genes (Springer, Berlin, 1967)
Muller, M. The relation of recombination to mutational advance. Mutat. Res. 1, 2–9 (1964)
Rice, W. R. Genetic hitch-hiking and the evolution of reduced genetic activity of the Y sex chromosome. Genetics 116, 161–167 (1987)
Skaletsky, H. et al. The male-specific region of the human Y chromosome is a mosaic of discrete sequence classes. Nature 423, 825–837 (2003)
Hofmeyr, J. D. J. Genetical studies of Carica papaya L. S. Afr. Dept Agric. Sci. Bull. 187, 64 (1938)
Storey, W. B. Segregation of sex types in Solo papaya and their application to the selection of seed. Am. Soc. Horticult. Sci. Proc. 35, 83–85 (1938)
Hofmeyr, J. D. J. Some genetic and breeding aspects of Carica papaya. Agronomia Tropical 17, 345–351 (1967)
Storey, W. B. in The Evolution of Crop Plants (ed. Simmonds, N. W.) 21–24 (Longman, London, 1976)
Horovitz, S. & Jiminez, H. Cruzamientos interespecificos e intergenericos en Caricaceaes y sus implicationes fitotecnias. Agronomia Tropical 17, 353–359 (1967)
Sondur, S. N., Manshardt, R. M. & Stiles, J. I. A genetic linkage map of papaya based on randomly amplified polymorphic DNA markers. Theor. Appl. Genet. 93, 547–553 (1996)
Ma, H. et al. High-density linkage mapping revealed suppression of recombination at the sex determination locus in papaya. Genetics (in the press)
Deputy, J. C. et al. Molecular marker for sex determination in papaya (Carica papaya L.). Theor. Appl. Genet. 106, 107–111 (2002)
Ming, R. et al. Construction and characterization of a papaya BAC library as a foundation for molecular dissection of a tree-fruit genome. Theor. Appl. Genet. 102, 892–899 (2001)
Storey, W. B. The botany and sex relations of the papaya. Hawaii Agricult. Exp. Station Bull 87, 5–22 (1941)
Tanksley, S. D., Miller, J. C., Paterson, A. H. & Bernatzky, R. Proc. 18th Stadler Genet. Symp. 157–173 (Plenum, New York, 1988)
Charlesworth, B. The evolution of sex chromosomes. Science 251, 1030–1033 (1991)
Lahn, B. T. & Page, D. C. Four evolutionary strata on the human X chromosome. Science 286, 964–967 (1999)
Iwase, M. et al. The amelogenin loci span an ancient pseudoautosomal boundary in diverse mammalian species. Proc. Natl Acad. Sci. USA 100, 5258–5263 (2003)
Charlesworth, D. Plant sex determination and sex chromosomes. Heredity 88, 94–101 (2002)
Filatov, D. A., Moneger, F., Negrutiu, I. & Charlesworth, D. Low variability in a Y-linked plant gene and its implications for Y-chromosome evolution. Nature 404, 388–390 (2000)
Chiu, C. T. Study on Sex Inheritance and Horticultural Characteristics of Hermaphrodite Papaya Thesis, Nat. Pingtung Univ. Sci. Technol., Taiwan (2000)
Storey, W. B. Genetics of the papaya. J. Hered. 44, 70–78 (1953)
Okada, S. et al. The Y chromosome in the liverwort Marchantia polymorpha has accumulated unique repeat sequences harboring a male-specific gene. Proc. Natl Acad. Sci. USA 98, 9454–9459 (2001)
Bachtrog, D. Adaptation shapes patterns of genome evolution on sexual and asexual chromosomes in Drosophila. Nature Genet. 34, 215–219 (2003)
Charlesworth, B. & Charlesworth, D. A model for the evolution of dioecy and gynodioecy. Am. Nat. 112, 975–997 (1978)
Yang, Z. N. & Mirkov, T. E. Isolation of large terminal sequences of BAC inserts based on double restriction enzyme digestion followed by anchored PCR. Genome 43, 412–415 (2000)
Siroky, J., Lysak, M. A., Dolezel, J., Kejnovsky, E. & Vyskot, B. Heterogeneity of rDNA distribution and genome size in Silene spp. Chromosome Res. 9, 387–393 (2001)
Lengerova, M., Moore, R. C., Grant, S. R. & Vyskot, B. The sex chromosomes of Silene latifolia revisited and revised. Genetics 165, 935–938 (2003)
Acknowledgements
We thank D. Charlesworth for comments on the manuscript; R. Perl-Treves for discussions; S. Ancheta, G. Asmus and L. Poland for technical assistance; R. Manshardt for providing an F2 population for fine-mapping; and H. Albert, M. Moore, R. Osgood, B. Vyskot and S. Whalen for reviewing the manuscript. This work was supported by a United States Department of Agriculture Agricultural Research Service (USDA-ARS) Cooperative Agreement with the Hawaii Agriculture Research Center, and a subaward to R. M. and A.H.P. to produce and to characterize the BAC library.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Supplementary information
41586_2004_BFnature02228_MOESM2_ESM.doc
Supplementary Figure 2: PCR amplification of duplicated cpsm90 and cpsm31 on BACs mapped to the tandem duplication region in the papaya MSY. (DOC 39 kb)
41586_2004_BFnature02228_MOESM3_ESM.doc
Supplementary Figure 3: Precocious separation of one pair of papaya chromosomes at anaphase I in pollen mother cells. (DOC 683 kb)
41586_2004_BFnature02228_MOESM4_ESM.doc
Supplementary Table: Comparison of male-specific DNA sequences amplified from papaya hermaphrodite and male genomic DNA. (DOC 24 kb)
Rights and permissions
About this article
Cite this article
Liu, Z., Moore, P., Ma, H. et al. A primitive Y chromosome in papaya marks incipient sex chromosome evolution. Nature 427, 348–352 (2004). https://doi.org/10.1038/nature02228
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature02228
This article is cited by
-
Evolution of a plant sex chromosome driven by expanding pericentromeric recombination suppression
Scientific Reports (2024)
-
Gene regulation network analyses of pistil development in papaya
BMC Genomics (2022)
-
Reinvention of hermaphroditism via activation of a RADIALIS-like gene in hexaploid persimmon
Nature Plants (2022)
-
Carica papaya: comprehensive overview of the nutritional values, phytochemicals and pharmacological activities
Advances in Traditional Medicine (2022)
-
Characterization and analysis of the promoter region of monodehydroascorbate reductase 4 (CpMDAR4) in papaya
Plant Reproduction (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.