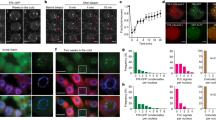
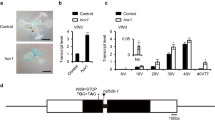
Abstract
In biennials and winter annuals, flowering is typically blocked in the first growing season. Exposure to the prolonged cold of winter, through a process called vernalization, is required to alleviate this block and permit flowering in the second growing season1. In winter-annual types of Arabidopsis thaliana, a flowering repressor, FLOWERING LOCUS C (FLC), is expressed at levels that inhibit flowering in the first growing season2. Vernalization promotes flowering by causing a repression of FLC that is mitotically stable after return to warm growing conditions2. Here we identify a gene with a function in the measurement of the duration of cold exposure and in the establishment of the vernalized state. We show that this silencing involves changes in the modification of histones in FLC chromatin.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Chouard, P. Vernalization and its relations to dormancy. Annu. Rev. Plant Physiol. 11, 191–238 (1960)
Michaels, S. & Amasino, R. Memories of winter: vernalization and the competence to flower. Plant Cell Environ. 23, 1145–1154 (2000)
Lee, I., Michaels, S. D., Masshardt, A. S. & Amasino, R. M. The late-flowering phenotype of FRIGIDA and LUMINIDEPENDENS is suppressed in the Landsberg erecta strain of Arabidopsis. Plant J. 6, 903–909 (1994)
Michaels, S. & Amasino, R. FLOWERING LOCUS C encodes a novel MADS domain protein that acts as a repressor of flowering. Plant Cell 11, 949–956 (1999)
Sheldon, C. C. et al. The FLF MADS box gene. A repressor of flowering in Arabidopsis regulated by vernalization and methylation. Plant Cell 11, 445–458 (1999)
Sheldon, C. C., Rouse, D. T., Finnegan, E. J., Peacock, W. J. & Dennis, E. S. The molecular basis of vernalization: the central role of FLOWERING LOCUS C (FLC). Proc. Natl Acad. Sci. USA 97, 3753–3758 (2000)
Michaels, S. D. & Amasino, R. M. Loss of FLOWERING LOCUS C activity eliminates the late-flowering phenotype of FRIGIDA and autonomous pathway mutations but not responsiveness to vernalization. Plant Cell 13, 935–942 (2001)
Johanson, U. et al. Molecular analysis of FRIGIDA, a major determinant of natural variation in Arabidopsis flowering time. Science 290, 344–347 (2000)
Gazzani, S., Gendall, A. R., Lister, C. & Dean, C. Analysis of the molecular basis of flowering time variation in Arabidopsis accessions. Plant Physiol. 132, 1107–1114 (2003)
Michaels, S. D., He, Y., Scortecci, K. C. & Amasino, R. M. Attenuation of FLOWERING LOCUS C activity as a mechanism for the evolution of summer-annual flowering behavior in Arabidopsis. Proc. Natl Acad. Sci. USA 100, 10102–10107 (2003)
Gendall, A. R., Levy, Y. Y., Wilson, A. & Dean, C. The VERNALIZATION 2 gene mediates the epigenetic regulation of vernalization in Arabidopsis. Cell 107, 525–535 (2001)
Levy, Y. Y., Mesnage, S., Mylne, J. S., Gendall, A. R. & Dean, C. Multiple roles of Arabidopsis VRN1 in vernalization and flowering time control. Science 297, 243–246 (2002)
Simpson, G. G. & Dean, C. Arabidopsis, the Rosetta stone of flowering time? Science 296, 285–289 (2002)
Fair, K. et al. Protein interactions of the MLL PHD fingers modulate MLL target gene regulation in human cells. Mol. Cell. Biol. 21, 3589–3597 (2001)
Main, A. L., Harvey, T. S., Baron, M., Boyd, J. & Campbell, I. D. The three-dimensional structure of the tenth type III module of fibronectin: an insight into RGD-mediated interactions. Cell 71, 671–678 (1992)
Wellensiek, S. J. Dividing cells as the locus for vernalization. Nature 195, 307–308 (1962)
Burn, J. E., Bagnall, D. J., Metzger, J. D., Dennis, E. S. & Peacock, W. J. DNA methylation, vernalization, and the initiation of flowering. Proc. Natl Acad. Sci. USA 90, 287–291 (1993)
Kuzmichev, A. R. D., Nishioka, K., Erdjument-Bromage, H., Tempst, P. & Reinberg, D. Histone methyltransferase activity associated with a human multiprotein complex containing the enhancer of Zeste protein. Genes Dev. 16, 2893–2905 (2002)
Richards, E. J. & Elgin, S. C. Epigenetic codes for heterochromatin formation and silencing: rounding up the usual suspects. Cell 108, 489–500 (2002)
Kehle, J. et al. dMi-2, a hunchback-interacting protein that functions in polycomb repression. Science 282, 1897–1900 (1998)
Sheldon, C. C., Conn, A. B., Dennis, E. S. & Peacock, W. J. Different regulatory regions are required for the vernalization-induced repression of FLOWERING LOCUS C and for the epigenetic maintenance of repression. Plant Cell 14, 2527–2537 (2002)
Lang, A. & Melchers, G. Vernalisation und Devernalisation bei einer zweijahrigen Pflanze. Z. Naturforsch 2b, 444–449 (1947)
Lang, A. in Encyclopedia of Plant Physiology Vol. 15 Part 1 (ed. Ruhland, W.) 1371–1536 (Springer, Berlin, 1965)
Reyes, J. C., Hennig, L. & Gruissem, W. Chromatin-remodeling and memory factors. New regulators of plant development. Plant Physiol. 130, 1090–1101 (2002)
Thomashow, M. F. So what's new in the field of plant cold acclimation? Lots! Plant Physiol. 125, 89–93 (2001)
Gozani, O. et al. The PHD finger of the chromatin-associated protein ING2 functions as a nuclear phosphoinositide receptor. Cell 114, 99–111 (2003)
Weigel, D. et al. Activation tagging in Arabidopsis. Plant Physiol. 122, 1003–1013 (2000)
Michaels, S. D. & Amasino, R. M. The gibberellic acid biosynthesis mutant ga1-3 of Arabidopsis thaliana is responsive to vernalization. Dev. Genet. 25, 194–198 (1999)
Michaels, S. D. et al. AGL24 acts as a promoter of flowering in Arabidopsis and is positively regulated by vernalization. Plant J. 33, 867–874 (2003)
Johnson, L., Cao, X. & Jacobsen, S. Interplay between two epigenetic marks. DNA methylation and histone H3 lysine 9 methylation. Curr. Biol. 12, 1360–1367 (2002)
Acknowledgements
We thank S. Michaels for creating and helping to screen the mutant populations that provided the vin3 mutant, for the FLC–GUS construct, and for advice and insight; and M. Doyle for help in the preparation of the manuscript. R.M.A. thanks C. O. Miller and J. A. D. Zeevaart for encouragement and mentoring in flowering-time regulation. This work was supported by the College of Agricultural and Life Sciences and the Graduate School of the University of Wisconsin, by the United States Department of Agriculture National Research Initiative Competitive Grants Program, and by a grant to R.M.A. from the National Science Foundation. The creation of insertion mutant lines was supported by a National Science Foundation grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Rights and permissions
About this article
Cite this article
Sung, S., Amasino, R. Vernalization in Arabidopsis thaliana is mediated by the PHD finger protein VIN3. Nature 427, 159–164 (2004). https://doi.org/10.1038/nature02195
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature02195
This article is cited by
-
Genome-wide identification and characterization of flowering genes in Citrus sinensis (L.) Osbeck: a comparison among C. Medica L., C. Reticulata Blanco, C. Grandis (L.) Osbeck and C. Clementina
BMC Genomic Data (2024)
-
Fine mapping and analysis of candidate genes for qBT2 and qBT7.2 locus controlling bolting time in radish (Raphanus sativus L.)
Theoretical and Applied Genetics (2024)
-
Development of SNP and InDel markers by genome resequencing and transcriptome sequencing in radish (Raphanus sativus L.)
BMC Genomics (2023)
-
Transcriptome analysis of critical genes related to flowering in Mikania micrantha at different altitudes provides insights for a potential control
BMC Genomics (2023)
-
Opening the gates
Nature Plants (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.