Abstract
Asexual reproduction by free-living invertebrate larvae is a rare and enigmatic phenomenon and, although it is known to occur in sea stars1,2,3,4 and brittle stars5,6, it has not been detected in other echinoderms despite more than a century of intensive study6,7. Here we describe spontaneous larval cloning in three species from two more echinoderm classes: a sea cucumber (Holothuroidea), a sand dollar and a sea urchin (Echinoidea). Larval cloning may therefore be an ancient ability of echinoderms and possibly of deutero-stomes — the group that includes echinoderms, acorn worms, sea squirts and vertebrates.
Similar content being viewed by others
Main
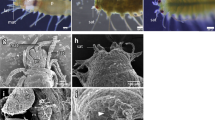
To confirm that genuine cloning was occurring, we reared cloning larvae and separated clones individually (Fig. 1, legend). Although most sea cucumber larvae metamorphosed normally after a month, 5 of 41 (12.2%) free-swimming doliolaria larvae were half the length of their siblings and had a constriction around the penultimate ciliary band. These buds retained a ciliary band and were still attached by a thin tether into the benthic pentacula stage 12 h later (Fig. 1a). Eventually, after separation, buds developed into normal auricularia larvae with a juvenile rudiment (Fig. 1b).
a, Sea cucumber (Parastichopus californicus; Holothuroidea; Aspidochirotida) early pentacula with clone attached at posterior end (arrowhead). b, Sea cucumber 18 days after separation as a complete auricularia larva, with juvenile rudiment (arrow). c, Sea urchin (Strongylocentrotus purpuratus; Echinoidea, Echinacea) larva with a clone (arrow) constricting from the posterior end around the robust ciliary band. d, Sea urchin clone three weeks after separation, with early juvenile rudiment (arrow). Scale bars, 50 μm. Adult P. californicus were collected sub-tidally from San Juan Island, Washington, and gametes were collected by dissection. Larvae were maintained at 11 °C under natural lighting in filtered sea water and were fed a red alga Rhodomonas salina, ad libitum. Adult S. purpuratus from Prasiola Point (Vancouver Island, Canada) were spawned by intracoelomic injection of 0.55 M potassium chloride. Larvae were maintained in a 19-h light cycle at 14 °C in pasteurized sea water and were fed > 106 cells per ml of a golden brown alga Isochrysis galbana and R. salina; food and water were replaced every 72 h. To monitor their development, cloning larvae and separated clones were isolated in 7-ml glass vials and cultured; most developed to metamorphosis. Cultures were deemed to be healthy as no larval tissue necrosis was seen. Further details are available from the authors.
Most sand dollar larvae metamorphosed within 7 weeks, but 6 of 170 (3.5%) formed a hollow bud near the future juvenile mouth (results not shown). Once separated, the evenly ciliated buds developed into an almost solid gastrula with bilateral spicules. Within two days, a tripartite gut and further skeletal spicules formed, and the clones began to feed.
After 4 weeks, nearly 5% of sea urchin larvae (n > 500) showed constrictions at their posterior end (Fig. 1c). These eventually yielded uniformly ciliated buds that began feeding within four days, developed paired skeletal rods within a week, and acquired a normal larval form within two weeks. Juvenile rudiments began to form within three weeks (Fig. 1d), accompanied by further larval arms.
Larval cloning is therefore known to occur in all classes except crinoids (feather stars and sea lilies), supporting an earlier conjecture that it might be an ancestral ability of echinoderms8. The mechanisms by which this cloning occurs, however, are unexpectedly diverse. First, clones may arise from various larval body regions, including arms (sea stars1,2,3,4, brittle stars5), the oral hood (sea stars2,3,4), the posterior end (sea stars3, sea cucumbers (Fig. 1a), sea urchins (Fig. 1c)) and the lateral body wall (sea stars2, sand dollars (our results, not shown)). Second, the developmental stage of clones at separation ranges from blastulae2 to fully formed larvae3. Third, some clones may not separate until after the primary larva has begun to metamorphose5 (Fig. 1a). This indicates either that larval cloning evolved independently on several occasions or that its mechanisms have diverged widely from an ancestral mode.
Cloning can be surprisingly frequent: up to 12% in laboratory-reared sea cucumber larvae and 10–90% in samples of field-collected sea star larvae1,4. The fact that such a common phenomenon should have been overlooked in heavily studied organisms seems remarkable. Were preconceptions about 'normal' development so strong that cloning was simply dismissed as aberrant? From a theoretical perspective, larval cloning — particularly in sea urchins — challenges a central tenet of the 'set-aside' cell theory9 because, contrary to prediction, larval body cells are not “essentially eutelic”9, but can differentiate into juvenile structures (Fig. 1b, d). Nevertheless, such cloning offers an opportunity to study the well-characterized developmental regulatory networks of sea urchins10 in a new ontogenetic context.
Larval cloning represents an intriguing new dimension to invertebrate life histories. The process confers three potential ecological advantages: increased fecundity under optimal growth conditions2,3, increased chances of settlement after a protracted larval life1,2,5, and recycling of otherwise discarded or reabsorbed larval tissue5. Larval cloning may also be evolutionarily significant, for two reasons. First, clones may subsequently clone5, potentially leading to a new, entirely pelagic bauplan. Second, although it may be merely another idiosyncrasy of echinoderm development, larval cloning might also be more taxonomically widespread. As nearest relatives to the echinoderms11, acorn worms offer a critical test. If their tornaria larvae clone, then ancient deuterosomes may have had this ability.
References
Bosh, I., Rivkin, R. B. & Alexander, S. P. Nature 337, 169–170 (1989).
Jaeckle, W. B. Biol. Bull. 186, 62–71 (1994).
Vickery, M. S. & McClintock, J. B. Biol. Bull. 199, 298–304 (2000).
Knott, K. E., Balser, E. J., Jaeckle, W. B. & Wray, G. A. Biol. Bull. 204, 246–255 (2003).
Balser, E. J. Biol. Bull. 194, 187–193 (1998).
Mortensen, T. Studies of the Development and Larval Forms of Echinoderms (Gad, Copenhagen, 1921).
MacBride, E. W. Nature 108, 529–530 (1921).
Lacalli, T. C. Invertebr. Biol. 119, 234–241 (2000).
Davidson, E. H., Cameron, R. A. & Ransick, A. Development 125, 3269–3290 (1998).
Davidson, E. H. et al. Science 295, 1669–1678 (2002).
Cameron, C. B., Garey, J. R. & Swalla, B. J. Proc. Natl Acad. Sci. USA 97, 4469–4474 (2000).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
brief communications is intended to provide a forum for brief, topical reports of general scientific interest and for technical discussion of recently published material of particular interest to non-specialist readers (communications arising). Priority will be given to contributions that have fewer than 500 words, 10 references and only one figure. Detailed guidelines are available on Nature's website (http://www.nature.com/nature).
Rights and permissions
About this article
Cite this article
Eaves, A., Palmer, A. Widespread cloning in echinoderm larvae. Nature 425, 146 (2003). https://doi.org/10.1038/425146a
Issue Date:
DOI: https://doi.org/10.1038/425146a
This article is cited by
-
Trioecy is maintained as a time-stable mating system in the pink sea urchin Toxopneustes roseus from the Mexican Pacific
Scientific Reports (2022)
-
Limited genetic signal from potential cloning and selfing within wild populations of coral-eating crown-of-thorns seastars (Acanthaster cf. solaris)
Coral Reefs (2021)
-
Interactive effects of temperature and salinity on early life stages of the sea urchin Heliocidaris crassispina
Marine Biology (2018)
-
An integrated view of asteroid regeneration: tissues, cells and molecules
Cell and Tissue Research (2017)
-
Bacterial communities of oceanic sea star (Asteroidea: Echinodermata) larvae
Marine Biology (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.