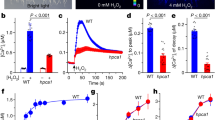
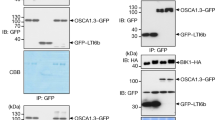
Abstract
Extracellular Ca2+ (Ca2+o) is required for various physiological and developmental processes in animals and plants1,2,3. In response to varied Ca2+o levels, plants maintain relatively constant internal Ca2+ content, suggesting a precise regulatory mechanism for Ca2+ homeostasis4. However, little is known about how plants monitor Ca2+o status and whether Ca2+o-sensing receptors exist. The effects of Ca2+o on guard cells in promoting stomatal closure by inducing increases in the concentration of cytosolic Ca2+ ([Ca2+]i)5,6,7,8 provide a clue to Ca2+o sensing. Here we have used a functional screening assay in mammalian cells9 to isolate an Arabidopsis complementary DNA clone encoding a Ca2+-sensing receptor, CAS. CAS is localized to the plasma membrane, exhibits low-affinity/high-capacity Ca2+ binding, and mediates Ca2+o-induced [Ca2+]i increases. CAS is expressed predominantly in the shoot, including guard cells. Repression of CAS disrupts Ca2+o signalling in guard cells, and impairs bolting (swift upward growth at the transition to seed production) in response to Ca2+ deficiency, so we conclude that CAS may be a primary transducer of Ca2+o in plants.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Brown, E. M. & MacLeod, R. J. Extracellular calcium sensing and extracellular calcium signaling. Physiol. Rev. 81, 239–297 (2001)
Hepler, P. K. & Wayne, R. O. Calcium and plant development. Annu. Rev. Plant Physiol. 36, 397–439 (1985)
Sanders, D., Pelloux, J., Brownlee, C. & Harper, J. F. Calcium at the crossroads of signaling. Plant Cell 14, S401–S417 (2002)
Loneragan, J. F. & Snowball, K. Calcium requirements of plants. Aust. J. Agr. Res. 20, 465–478 (1969)
MacRobbie, E. Calcium and ABA-induced stomatal closure. Phil. Trans. R. Soc. Lond. B 338, 5–18 (1992)
McAinsh, M. R., Webb, A. A. R., Taylor, J. E. & Hetherington, A. M. Stimulus-induced oscillations in guard cell cytosolic free calcium. Plant Cell 7, 1207–1219 (1995)
Allen, G. J. et al. A defined range of guard cell calcium oscillation parameters encodes stomatal movements. Nature 411, 1053–1057 (2001)
Schroeder, J. I., Kwak, J. M. & Allen, G. J. Guard cell abscisic acid signalling and engineering drought hardiness in plants. Nature 410, 327–330 (2001)
Caterina, M. J. et al. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389, 816–824 (1997)
Clarkson, D. T. Calcium transport between tissues and its distribution in the plant. Plant Cell Environ. 7, 449–456 (1984)
Luan, S., Kudla, J., Rodriguez-Concepcion, M., Yalovsky, S. & Gruissem, W. Calmodulins and calcineurin B-like proteins: calcium sensors for specific signal response coupling in plants. Plant Cell 14, S389–S400 (2002)
Schwartz, A. Role of Ca2+ and EGTA on stomatal movements in Commelina communis L. Plant Physiol. 79, 1003–1005 (1985)
Berridge, M. J., Bootman, M. D. & Lipp, P. Calcium—a life and death signal. Nature 395, 645–648 (1998)
Sattelmacher, B. The apoplast and its significance for plant mineral nutrition. New Phytol. 149, 167–192 (2001)
Chapman, E. R. Synaptotagmin: a Ca2+ sensor that triggers exocytosis? Nature Rev. Mol. Cell Biol. 3, 498–508 (2002)
Pei, Z.-M., Baizabal-Aguirre, V. M., Allen, G. J. & Schroeder, J. I. A transient outward-rectifying K+ channel current down-regulated by cytosolic Ca2+ in Arabidopsis thaliana guard cells. Proc. Natl Acad. Sci. USA 95, 6548–6553 (1998)
Friml, J., Wisniewska, J., Benkova, E., Mendgen, K. & Palme, K. Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature 415, 806–809 (2002)
Pei, Z.-M. et al. Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 406, 731–734 (2000)
Simpson, G. G. & Dean, C. Arabidopsis, the Rosetta stone of flowering time? Science 296, 285–289 (2002)
Gilroy, S., Read, N. D. & Trewavas, A. J. Elevation of cytoplasmic Ca2+ by caged calcium or caged inositol triphosphate initiates stomatal closure. Nature 346, 769–771 (1990)
Staxen, I. et al. Abscisic acid induces oscillations in guard-cell cytosolic free calcium that involve phosphoinositide-specific phospholipase C. Proc. Natl Acad. Sci. USA 96, 1779–1784 (1999)
Roelfsema, M. R. G. & Hedrich, R. Studying guard cells in the intact plant: modulation of stomatal movement by apoplastic factors. New Phytol. 153, 425–431 (2002)
Maruyama, K., Mikawa, T. & Ebashi, S. Detection of calcium binding proteins by 45Ca autoradiography on nitrocellulose membrane after sodium dodecyl sulfate gel electrophoresis. J. Biochem. 95, 511–519 (1984)
Wakabayashi, H., Schmidt, K. M. & Fay, P. J. Ca2+ binding to both the heavy and light chains of factor VIII is required for cofactor activity. Biochemistry 41, 8485–8492 (2002)
Li, X., Zhang, Y., Clarke, J. D., Li, Y. & Dong, X. Identification and cloning of a negative regulator of systemic acquired resistance, SNI1, through a screen for suppressors of npr1-1. Cell 98, 329–339 (1999)
Clough, S. J. & Bent, A. F. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16, 735–743 (1998)
Acknowledgements
We thank L. Jing, Y. Hao and Y. He for assistance; W. Zhang for HEK293 cells and discussions on expression cloning; D. McClay and R. Fehon for use of cell culture and imaging facilities; J. Schroeder, J. Kwak, J. Harper and A. VanDogen for vectors; J. Schroeder, T. Sun and R. Venters for discussions; and J. Siedow, X. Dong, W. Durrant, P. Benfey, W. Zhang and R. Fehon for comments on the manuscript. R.T. was supported in part by a Hargitt Fellowship. This work was supported by start-up funds from Duke University and by a grant from the NSF to Z.-M.P.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Han, S., Tang, R., Anderson, L. et al. A cell surface receptor mediates extracellular Ca2+ sensing in guard cells. Nature 425, 196–200 (2003). https://doi.org/10.1038/nature01932
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature01932
This article is cited by
-
A pyrenoid-localized protein SAGA1 is necessary for Ca2+-binding protein CAS-dependent expression of nuclear genes encoding inorganic carbon transporters in Chlamydomonas reinhardtii
Photosynthesis Research (2023)
-
Calcium signatures and signal transduction schemes during microbe interactions in Arabidopsis thaliana
Journal of Plant Biochemistry and Biotechnology (2020)
-
Exploring the adaptive mechanism of Passiflora edulis in karst areas via an integrative analysis of nutrient elements and transcriptional profiles
BMC Plant Biology (2019)
-
Comparative transcriptomic analysis reveals common molecular factors responsive to heat and drought stress in Agrostis stolonifera
Scientific Reports (2018)
-
Ca2+ channels and Ca2+ signals involved in abiotic stress responses in plant cells: recent advances
Plant Cell, Tissue and Organ Culture (PCTOC) (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.