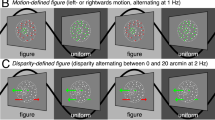
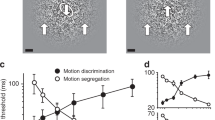
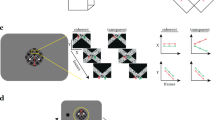
Abstract
An important task in vision is to detect objects moving within a stationary scene. During normal viewing this is complicated by the presence of eye movements that continually scan the image across the retina, even during fixation. To detect moving objects, the brain must distinguish local motion within the scene from the global retinal image drift due to fixational eye movements. We have found that this process begins in the retina: a subset of retinal ganglion cells responds to motion in the receptive field centre, but only if the wider surround moves with a different trajectory. This selectivity for differential motion is independent of direction, and can be explained by a model of retinal circuitry that invokes pooling over nonlinear interneurons. The suppression by global image motion is probably mediated by polyaxonal, wide-field amacrine cells with transient responses. We show how a population of ganglion cells selective for differential motion can rapidly flag moving objects, and even segregate multiple moving objects.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Yarbus, A. L. Eye Movements and Vision (Plenum, New York, 1967)
Kowler, E. Eye Movements and their Role in Visual and Cognitive Processes (Elsevier, New York, 1990)
Ross, J., Morrone, M. C., Goldberg, M. E. & Burr, D. C. Changes in visual perception at the time of saccades. Trends Neurosci. 24, 113–121 (2001)
Coppola, D. & Purves, D. The extraordinarily rapid disappearance of entopic images. Proc. Natl Acad. Sci. USA 93, 8001–8004 (1996)
Skavenski, A. A., Hansen, R. M., Steinman, R. M. & Winterson, B. J. Quality of retinal image stabilization during small natural and artificial body rotations in man. Vision Res. 19, 675–683 (1979)
Manteuffel, G., Plasa, L., Sommer, T. J. & Wess, O. Involuntary eye movements in salamanders. Naturwissenschaften 64, 533–534 (1977)
Van der Steen, J. & Collewijn, H. Ocular stability in the horizontal, frontal and sagittal planes in the rabbit. Exp. Brain Res. 56, 263–274 (1984)
Gibson, J. J. The Perception of the Visual World (Houghton Mifflin, Boston, 1950)
Vernon, M. D. The Psychology of Perception (Penguin, Baltimore, Maryland, 1962)
Graham, C. H. in Vision and Visual Perception (ed. Graham, C. H.) 575–588 (Wiley, New York, 1965)
Hammond, P. & Smith, A. T. On the sensitivity of complex cells in feline striate cortex to relative motion. Exp. Brain Res. 47, 457–460 (1982)
Born, R. T. & Tootell, R. B. Segregation of global and local motion processing in primate middle temporal visual area. Nature 357, 497–499 (1992)
Bender, D. B. & Davidson, R. M. Global visual processing in the monkey superior colliculus. Brain Res. 381, 372–375 (1986)
Sterling, P. & Wickelgren, B. G. Visual receptive fields in the superior colliculus of the cat. J. Neurophysiol. 32, 1–15 (1969)
Frost, B. J. & Nakayama, K. Single visual neurons code opposing motion independent of direction. Science 220, 744–745 (1983)
Steinman, R. M. & Collewijn, H. Binocular retinal image motion during active head rotation. Vision Res. 20, 415–429 (1980)
Cook, P. B., Lukasiewicz, P. D. & McReynolds, J. S. Action potentials are required for the lateral transmission of glycinergic transient inhibition in the amphibian retina. J. Neurosci. 18, 2301–2308 (1998)
Werblin, F. S. Lateral interactions at inner plexiform layer of vertebrate retina: antagonistic responses to change. Science 175, 1008–1010 (1972)
Enroth-Cugell, C. & Jakiela, H. G. Suppression of cat retinal ganglion cell responses by moving patterns. J. Physiol. 302, 49–72 (1980)
Passaglia, C. L., Enroth-Cugell, C. & Troy, J. B. Effects of remote stimulation on the mean firing rate of cat retinal ganglion cells. J. Neurosci. 21, 5794–5803 (2001)
Werblin, F., Maguire, G., Lukasiewicz, P., Eliasof, S. & Wu, S. M. Neural interactions mediating the detection of motion in the retina of the tiger salamander. Visual Neurosci. 1, 317–329 (1988)
Famiglietti, E. V. Polyaxonal amacrine cells of rabbit retina: size and distribution of PA1 cells. J. Comp. Neurol. 316, 406–421 (1992)
Volgyi, B., Xin, D., Amarillo, Y. & Bloomfield, S. A. Morphology and physiology of the polyaxonal amacrine cells in the rabbit retina. J. Comp. Neurol. 440, 109–125 (2001)
Dacey, D. M. Axon-bearing amacrine cells of the macaque monkey retina. J. Comp. Neurol. 284, 275–293 (1989)
Stafford, D. K. & Dacey, D. M. Physiology of the A1 amacrine: a spiking, axon-bearing interneuron of the macaque monkey retina. Vis. Neurosci. 14, 507–522 (1997)
Grossman, G. E., Leigh, R. J., Bruce, E. N., Huebner, W. P. & Lanska, D. J. Performance of the human vestibuloocular reflex during locomotion. J. Neurophysiol. 62, 264–272 (1989)
Hochstein, S. & Shapley, R. M. Linear and nonlinear spatial subunits in Y cat retinal ganglion cells. J. Physiol. 262, 265–284 (1976)
Shapley, R. M. & Victor, J. D. Nonlinear spatial summation and the contrast gain control of cat retinal ganglion cells. J. Physiol. 290, 141–161 (1979)
Victor, J. D. & Shapley, R. M. The nonlinear pathway of Y ganglion cells in the cat retina. J. Gen. Physiol. 74, 671–689 (1979)
Demb, J. B., Zaghloul, K., Haarsma, L. & Sterling, P. Bipolar cells contribute to nonlinear spatial summation in the brisk-transient (Y) ganglion cell in mammalian retina. J. Neurosci. 21, 7447–7454 (2001)
Wu, S. M., Gao, F. & Maple, B. R. Functional architecture of synapses in the inner retina: segregation of visual signals by stratification of bipolar cell axon terminals. J. Neurosci. 20, 4462–4470 (2000)
Greschner, M., Bongard, M., Rujan, P. & Ammermuller, J. Retinal ganglion cell synchronization by fixational eye movements improves feature estimation. Nature Neurosci. 5, 341–347 (2002)
Regan, D. Human Perception of Objects: Early Visual Processing of Spatial Form Defined by Luminance, Color, Texture, Motion, and Binocular Disparity (Sinauer, Sunderland, Massachusetts, 2000)
Singer, W. Neuronal synchrony: a versatile code for the definition of relations? Neuron 24, 49–65 (1999)
Blakemore, C. & Vital-Durand, F. Organization and post-natal development of the monkey's lateral geniculate nucleus. J. Physiol. 380, 453–491 (1986)
Kaplan, E. & Shapley, R. M. The primate retina contains two types of ganglion cells, with high and low contrast sensitivity. Proc. Natl Acad. Sci. USA 83, 2755–2757 (1986)
Shapley, R., Kaplan, E. & Soodak, R. Spatial summation and contrast sensitivity of X and Y cells in the lateral geniculate nucleus of the macaque. Nature 292, 543–545 (1981)
Ouchi, H. Japanese Optical and Geometrical Art (Dover, New York, 1977)
Meister, M., Pine, J. & Baylor, D. A. Multi-neuronal signals from the retina: acquisition and analysis. J. Neurosci. Methods 51, 95–106 (1994)
Baccus, S. A. & Meister, M. Fast and slow contrast adaptation in retinal circuitry. Neuron 36, 909–919 (2002)
Warland, D. K., Reinagel, P. & Meister, M. Decoding visual information from a population of retinal ganglion cells. J. Neurophysiol. 78, 2336–2350 (1997)
DeVries, S. H. Correlated firing in rabbit retinal ganglion cells. J. Neurophysiol. 81, 908–920 (1999)
Chichilnisky, E. J. A simple white noise analysis of neuronal light responses. Network 12, 199–213 (2001)
Acknowledgements
We thank members of the Meister laboratory for advice; P. Cavanagh, F. Engert, V. Murthy and K. Nakayama for comments on the manuscript; and H. van der Steen for providing the eye movement data in Fig. 1b. This work was supported by a grant from NEI (M.M.) and NRSA (S.A.B.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Rights and permissions
About this article
Cite this article
Ölveczky, B., Baccus, S. & Meister, M. Segregation of object and background motion in the retina. Nature 423, 401–408 (2003). https://doi.org/10.1038/nature01652
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature01652
This article is cited by
-
A presynaptic source drives differing levels of surround suppression in two mouse retinal ganglion cell types
Nature Communications (2024)
-
Neuro-inspired optical sensor array for high-accuracy static image recognition and dynamic trace extraction
Nature Communications (2023)
-
Modular interneuron circuits control motion sensitivity in the mouse retina
Nature Communications (2023)
-
CMOS-compatible retinomorphic Si photodetector for motion detection
Science China Information Sciences (2023)
-
Two-dimensional materials-based integrated hardware
Science China Information Sciences (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.