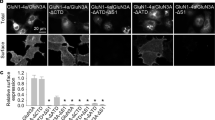
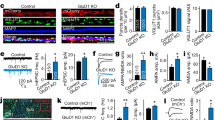
Abstract
NMDA (N-methyl-d-aspartate) receptors (NMDARs) are a principal subtype of excitatory ligand-gated ion channel with prominent roles in physiological and disease processes in the central nervous system1. Recognition that glycine potentiates NMDAR-mediated currents2 as well as being a requisite co-agonist of the NMDAR subtype of ‘glutamate’ receptor3 profoundly changed our understanding of chemical synaptic communication in the central nervous system. The binding of both glycine and glutamate is necessary to cause opening of the NMDAR conductance pore1. Although binding of either agonist alone is insufficient to cause current flow through the channel, we report here that stimulation of the glycine site initiates signalling through the NMDAR complex, priming the receptors for clathrin-dependent endocytosis. Glycine binding alone does not cause the receptor to be endocytosed; this requires both glycine and glutamate site activation of NMDARs. The priming effect of glycine is mimicked by the NMDAR glycine site agonist d-serine, and is blocked by competitive glycine site antagonists. Synaptic as well as extrasynaptic NMDARs are primed for internalization by glycine site stimulation. Our results demonstrate transmembrane signal transduction through activating the glycine site of NMDARs, and elucidate a model for modulating cell–cell communication in the central nervous system.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Dingledine, R., Borges, K., Bowie, D. & Traynelis, S. F. The glutamate receptor ion channels. Pharmacol. Rev. 51, 7–61 (1999)
Johnson, J. W. & Ascher, P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature 325, 529–531 (1987)
Kleckner, N. W. & Dingledine, R. Requirement for glycine in activation of NMDA-receptors expressed in Xenopus oocytes. Science 241, 835–837 (1988)
Carroll, R. C., Lissin, D. V., von Zastrow, M., Nicoll, R. A. & Malenka, R. C. Rapid redistribution of glutamate receptors contributes to long-term depression in hippocampal cultures. Nature Neurosci. 2, 454–460 (1999)
Lissin, D. V. et al. Activity differentially regulates the surface expression of synaptic AMPA and NMDA glutamate receptors. Proc. Natl Acad. Sci. USA 95, 7097–7102 (1998)
Man, H. Y. et al. Regulation of AMPA receptor-mediated synaptic transmission by clathrin-dependent receptor internalization. Neuron 25, 649–662 (2000)
Ehlers, M. D. Reinsertion or degradation of AMPA receptors determined by activity-dependent endocytic sorting. Neuron 28, 511–525 (2000)
Luscher, C. et al. Role of AMPA receptor cycling in synaptic transmission and plasticity. Neuron 24, 649–658 (1999)
Carroll, R. C. et al. Dynamin-dependent endocytosis of ionotropic glutamate receptors. Proc. Natl Acad. Sci. USA 96, 14112–14117 (1999)
Kittler, J. T. et al. Constitutive endocytosis of GABAA receptors by an association with the adaptin AP2 complex modulates inhibitory synaptic currents in hippocampal neurons. J. Neurosci. 20, 7972–7977 (2000)
Roche, K. W. et al. Molecular determinants of NMDA receptor internalization. Nature Neurosci. 4, 794–802 (2001)
Vissel, B., Krupp, J. J., Heinemann, S. F. & Westbrook, G. L. A use-dependent tyrosine dephosphorylation of NMDA receptors is independent of ion flux. Nature Neurosci. 4, 587–596 (2001)
Snyder, E. M. et al. Internalization of ionotropic glutamate receptors in response to mGluR activation. Nature Neurosci. 4, 1079–1085 (2001)
Shupliakov, O. et al. Synaptic vesicle endocytosis impaired by disruption of dynamin-SH3 domain interactions. Science 276, 259–263 (1997)
Grabs, D. et al. The SH3 domain of amphiphysin binds the proline-rich domain of dynamin at a single site that defines a new SH3 binding consensus sequence. J. Biol. Chem. 272, 13419–13425 (1997)
Hansen, S. H., Sandvig, K. & van Deurs, B. Clathrin and HA2 adaptors: effects of potassium depletion, hypertonic medium, and cytosol acidification. J. Cell Biol. 121, 61–72 (1993)
Watkins, J. C. & Olverman, H. J. Agonists and antagonists for excitatory amino acid receptors. Trends Neurosci. 10, 265–272 (1987)
Mothet, J. P. et al. D-serine is an endogenous ligand for the glycine site of the N-methyl-D-aspartate receptor. Proc. Natl Acad. Sci. USA 97, 4926–4931 (2000)
Foster, A. C. et al. Kynurenic acid analogues with improved affinity and selectivity for the glycine site on the N-methyl-D-aspartate receptor from rat brain. Mol. Pharmacol. 41, 914–922 (1992)
Danysz, W. & Parsons, C. G. Glycine and N-methyl-D-aspartate receptors: physiological significance and possible therapeutic applications. Pharmacol. Rev. 50, 597–664 (1998)
Slepnev, V. I. & De Camilli, P. Accessory factors in clathrin-dependent synaptic vesicle endocytosis. Nature Rev. Neurosci. 1, 161–172 (2000)
Salituro, F. G. et al. 3-(2-Carboxyindol-3-yl)propionic acid-based antagonists of the N-methyl-D-aspartic acid receptor associated glycine binding site. J. Med. Chem. 35, 1791–1799 (1992)
Marks, B. & McMahon, H. T. Calcium triggers calcineurin-dependent synaptic vesicle recycling in mammalian nerve terminals. Curr. Biol. 8, 740–749 (1998)
Bekkers, J. M. & Stevens, C. F. NMDA and non-NMDA receptors are co-localized at individual excitatory synapses in cultured rat hippocampus. Nature 341, 230–233 (1989)
Mugnaini, M., Dal Forno, G., Corsi, M. & Bunnemann, B. Receptor binding characteristics of the novel NMDA receptor glycine site antagonist [3H]GV150526A in rat cerebral cortical membranes. Eur. J. Pharmacol. 391, 233–241 (2000)
Mugnaini, M., Antolini, M., Corsi, M. & van Amsterdam, F. T. [3H]5,7-dichlorokynurenic acid recognizes two binding sites in rat cerebral cortex membranes. J. Recept. Signal. Transduct. Res. 18, 91–112 (1998)
Popik, P. et al. [3H]1-aminocyclopropanecarboxylic acid, a novel probe for strychnine-insensitive glycine receptors. Eur. J. Pharmacol. 291, 221–227 (1995)
Colquhoun, D. Binding, gating, affinity and efficacy: the interpretation of structure-activity relationships for agonists and of the effects of mutating receptors. Br. J. Pharmacol. 125, 924–947 (1998)
Huang, Y. et al. CAKbeta/Pyk2 kinase is a signaling link for induction of long-term potentiation in CA1 hippocampus. Neuron 29, 485–496 (2001)
Acknowledgements
We thank J. L. Hicks and Y. Li for technical support, and J. F. MacDonald and L. Y. Wang for comments on the manuscript. This work was supported by the Canadian Institutes of Health Research (CIHR; to M.W.S. and Y.T.W.), the EJLB Foundation (to Y.T.W.), the Ontario Neurotrauma Foundation (ONF; to M.W.S.) and the Nicole Fealdman Memorial Fund (to M.W.S.). M.W.S. is a CIHR Investigator, Y.T.W. is a CIHR Investigator and the holder of the HSF of British Columbia and Yukon Chair in Stroke Research at the University of British Columbia and Vancouver Hospital and Health Sciences Centre, and is an International Scholar of the Howard Hughes Medical Institute. Y.N. is a Ronald Melzack Fellow of CIHR; Y.Q.H. is a Fellow of the ONF and CIHR/Heart and Stroke Foundation of Canada (HSFC); G.A. is a Fellow of Research Training Centre at Hospital for Sick Children; W.J. is a CIHR/HSFC student; and L.V.K. is a CIHR MD/PhD student. We thank J. F. MacDonald and M. Sheng for providing cDNA constructs. Y.T.W. and M.W.S. are joint senior authors.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Rights and permissions
About this article
Cite this article
Nong, Y., Huang, YQ., Ju, W. et al. Glycine binding primes NMDA receptor internalization. Nature 422, 302–307 (2003). https://doi.org/10.1038/nature01497
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature01497
This article is cited by
-
STIM2 regulates NMDA receptor endocytosis that is induced by short-term NMDA receptor overactivation in cortical neurons
Cellular and Molecular Life Sciences (2023)
-
Enhanced fear limits behavioral flexibility in Shank2-deficient mice
Molecular Autism (2022)
-
Bupivacaine reduces GlyT1 expression by potentiating the p-AMPKα/BDNF signalling pathway in spinal astrocytes of rats
Scientific Reports (2022)
-
Shenzhi Jiannao formula ameliorates vascular dementia in vivo and in vitro by inhibition glutamate neurotoxicity via promoting clathrin-mediated endocytosis
Chinese Medicine (2021)
-
Tripartite signalling by NMDA receptors
Molecular Brain (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.