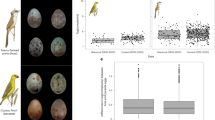
Abstract
Cuckoo nestlings that evict all other young from the nest soon after hatching impose a high reproductive cost on their hosts1. In defence, hosts have coevolved strategies to prevent brood parasitism. Puzzlingly, they do not extend beyond the egg stage2,3,4,5. Thus, hosts adept at recognizing foreign eggs remain vulnerable to exploitation by cuckoo nestlings6,7. Here we show that the breach of host egg defences by cuckoos creates a new stage in the coevolutionary cycle. We found that defences used during the egg-laying period by host superb fairy-wrens (Malurus cyaneus) are easily evaded by the Horsfield's bronze-cuckoo (Chrysococcyx basalis), a specialist fairy-wren brood parasite. However, although hosts never deserted their own broods, they later abandoned 40% of nests containing a lone Horsfield's bronze-cuckoo nestling, and 100% of nests with a lone shining bronze-cuckoo nestling (Chrysococcyx lucidus), an occasional fairy-wren brood parasite. Our experiments demonstrate that host discrimination against evictor-cuckoo nestlings is possible, and suggest that it has selected for the evolution of nestling mimicry in bronze-cuckoos.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Davies, N. B. Cuckoos, Cowbirds and Other Cheats (Poyser, London, 2000)
Brooke, M. de L. & Davies, N. B. Egg mimicry by cuckoos Cuculus canorus in relation to discrimination by hosts. Nature 335, 630–632 (1988)
Davies, N. B. & Brooke, M. de L. Cuckoos versus reed warblers: adaptations and counteradaptations. Anim. Behav. 36, 262–284 (1988)
Lotem, A., Nakamura, H. & Zahavi, A. Constraints on egg discrimination and cuckoo host coevolution. Anim. Behav. 49, 1185–1209 (1995)
Marchetti, K. Egg rejection in a passerine bird: size does matter. Anim. Behav. 59, 877–883 (2000)
Lotem, A. Learning to recognize nestlings is maladaptive for cuckoo Cuculus canorus hosts. Nature 362, 743–745 (1993)
Kilner, R. M., Noble, D. G. & Davies, N. B. Signals of need in parent–offspring communication and their exploitation by the common cuckoo. Nature 397, 667–672 (1999)
Brooker, M. G. & Brooker, L. C. The comparative breeding behaviour of two sympatric cuckoos, Horsfield's bronze-cuckoo Chrysococcyx basalis and the shining bronze-cuckoo C. lucidus, in Western Australia: a new model for the evolution of egg morphology and host specificity in avian brood parasites. Ibis 131, 528–547 (1989)
Mason, P. & Rothstein, S. I. Coevolution and avian brood parasitism: Cowbird eggs show evolutionary response to host discrimination. Evolution 40, 1207–1214 (1986)
Krüger, O. & Davies, N. B. The evolution of cuckoo parasitism: a comparative analysis. Proc. R. Soc. Lond. B 269, 375–381 (2002)
Davies, N. B. & Brooke, M. de L. An experimental study of co-evolution between the cuckoo, Cuculus canorus, and its hosts. II. Host egg markings, chick discrimination and general discussion. J. Anim. Ecol. 58, 225–236 (1989)
Davies, N. B., Brooke, M. de L. & Kacelnik, A. Recognition errors and probability of parasitism determine whether reed warblers should accept or reject mimetic cuckoo eggs. Proc. R. Soc. Lond. B 263, 925–931 (1996)
Lotem, A. & Nakamura, H. in Parasitic Birds and Their Hosts. Studies in Coevolution (eds Rothstein, S. I. & Robinson, S. K.) 223–235 (Oxford Univ. Press, Oxford, 1998)
Mulder, R. A. Evolutionary Ecology of the Mating System of Superb Fairy-wrens. PhD thesis, Australian National Univ. (1992)
Fraga, R. M. in Parasitic Birds and their Hosts. Studies in Coevolution (eds Rothstein, S. I. & Robinson, S. K.) 173–193 (Oxford Univ. Press, Oxford, 1998)
Lichtenstein, G. Low success of shiny cowbird chicks parasitizing rufous-bellied thrushes: chick-chick competition or parental discrimination? Anim. Behav. 61, 401–413 (2001)
Payne, R. B., Woods, J. L. & Payne, L. L. Parental care in estrildid finches: experimental tests of a model of Vidua brood parasitism. Anim. Behav. 62, 473–483 (2001)
Mulder, R. A., Dunn, P. O., Cockburn, A., Lazenby-Cohen, K. A. & Howell, M. J. Helpers liberate female fairy-wrens from constraints on extra-pair mate choice. Proc. R. Soc. Lond. B 255, 223–229 (1994)
Payne, R. B. & Payne, L. L. in Parasitic Birds and their Hosts. Studies in Coevolution (eds Rothstein, S. I. & Robinson, S. K.) 152–169 (Oxford Univ. Press, Oxford, 1998)
Tidemann, S. C. The Behavioural Ecology of Three Coexisting Fairy-wrens (Maluridae: Malurus). PhD thesis, Australian National Univ. (1983)
Nias, R. C. Co-operative Breeding in the Superb Fairy-wren Malurus cyaneus. PhD thesis, Univ. New England (1987)
Rowley, I. & Russell, E. Fairy-wrens and Grasswrens (Oxford Univ. Press, Oxford, 1997)
Ogilvie, M. A. in Handbook of the Birds of Europe, the Middle East and North Africa, The Birds of the Western Palearctic Vol. VI (eds Cramp, S. & Brooks, D. J.) 193–212 (Oxford Univ. Press, Oxford, 1992)
Bibby, C. J. Some breeding statistics of Reed and Sedge Warblers. Bird Study 25, 207–222 (1978)
Rowley, I. The life history of the superb blue wren Malurus cyaneus. Emu 64, 251–297 (1965)
Brooker, M. G. & Brooker, L. C. Cuckoo hosts in Australia. Aust. Zool. Rev. 2, 1–67 (1989)
Cuthill, I. C., Bennett, A. T. D., Partridge, J. C. & Meier, E. J. Plumage reflectance and the objective assessment of avian sexual dichromatism. Am. Nat. 153, 183–200 (1999)
Acknowledgements
N.E.L. was supported by an Australian Research Council Australian Postdoctoral Fellowship. R.M.K. was supported by a Royal Society Dorothy Hodgkin Research Fellowship and a Royal Society University Research Fellowship. Spectrophotometric equipment was purchased with a BBSRC grant. We thank Environment Australia for allowing us to work at Campbell Park; S. Butchart, A. Cockburn, N. Davies, M. Hall, G. Maurer, J. Madden, N. Macgregor and A. Peters for assistance in the field; A. Cockburn for statistical advice; and M. de la Brooke, A. Cockburn, N. Davies, R. Heinsohn, C. Hinde, A. Lotem, R. Magrath and S. Rothstein for comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Rights and permissions
About this article
Cite this article
Langmore, N., Hunt, S. & Kilner, R. Escalation of a coevolutionary arms race through host rejection of brood parasitic young. Nature 422, 157–160 (2003). https://doi.org/10.1038/nature01460
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature01460
This article is cited by
-
Nest Integration: a novel form of food acquisition by altricial fledglings
Evolutionary Ecology (2023)
-
Eggshell spots are an important cue for the egg retrieval behavior in two tit species
Animal Cognition (2023)
-
Fledgling discrimination in the hoopoe, a potential host species of the great spotted cuckoo
Behavioral Ecology and Sociobiology (2023)
-
Thicker eggshells are not predicted by host egg ejection behaviour in four species of Australian cuckoo
Scientific Reports (2022)
-
Nestling odour modulates behavioural response in male, but not in female zebra finches
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.