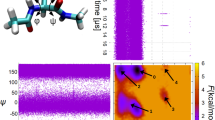
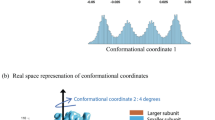
Abstract
Combining experimental and simulation data to describe all of the structures and the pathways involved in folding a protein is problematical. Transition states can be mapped experimentally by φ values1,2, but the denatured state3 is very difficult to analyse under conditions that favour folding. Also computer simulation at atomic resolution is currently limited to about a microsecond or less. Ultrafast-folding proteins fold and unfold on timescales accessible by both approaches4,5, so here we study the folding pathway of the three-helix bundle protein Engrailed homeodomain6. Experimentally, the protein collapses in a microsecond to give an intermediate with much native α-helical secondary structure, which is the major component of the denatured state under conditions that favour folding. A mutant protein shows this state to be compact and contain dynamic, native-like helices with unstructured side chains. In the transition state between this and the native state, the structure of the helices is nearly fully formed and their docking is in progress, approximating to a classical diffusion–collision model. Molecular dynamics simulations give rate constants and structural details highly consistent with experiment, thereby completing the description of folding at atomic resolution.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Fersht, A. R., Leatherbarrow, R. J. & Wells, T. N. Structure–activity relationships in engineered proteins: analysis of use of binding energy by linear free energy relationships. Biochemistry 26, 6030–6038 (1987)
Fersht, A. R., Matouschek, A. & Serrano, L. The folding of an enzyme. I. Theory of protein engineering analysis of stability and pathway of protein folding. J. Mol. Biol. 224, 771–782 (1992)
Shortle, D. The denatured state (the other half of the folding equation) and its role in protein stability. FASEB J. 10, 27–34 (1996)
Mayor, U., Johnson, C. M., Daggett, V. & Fersht, A. R. Protein folding and unfolding in microseconds to nanoseconds by experiment and simulation. Proc. Natl Acad. Sci. USA 97, 13518–13522 (2000)
Ferguson, N. et al. Using flexible loop mimetics to extend phi-value analysis to secondary structure interactions. Proc. Natl Acad. Sci. USA 98, 13008–13013 (2001)
Clarke, N. D., Kissinger, C. R., Desjarlais, J., Gilliland, G. L. & Pabo, C. O. Structural studies of the engrailed homeodomain. Prot. Sci. 3, 1779–1787 (1994)
Blanco, F. J., Serrano, L. & Forman-Kay, J. D. High populations of non-native structures in the denatured state are compatible with the formation of the native folded state. J. Mol. Biol. 284, 1153–1164 (1998)
Spector, S., Rosconi, M. & Raleigh, D. P. Conformational analysis of peptide fragments derived from the peripheral subunit-binding domain from the pyruvate dehydrogenase multienzyme complex of Bacillus stearothermophilus: evidence for nonrandom structure in the unfolded state. Biopolymers 49, 29–40 (1999)
Serrano, L. & Fersht, A. R. Principles of protein stability derived from protein engineering methods. Curr. Opin. Struct. Biol. 3, 75–83 (1993)
Englander, S. W. & Krishna, M. M. G. Hydrogen exchange. Nature Struct. Biol. 8, 741–742 (2001)
Oliveberg, M. & Fersht, A. R. Thermodynamics of transient conformations in the folding pathway of barnase: reorganization of the folding intermediate at low pH. Biochemistry 35, 2738–2749 (1996)
Thompson, P. A., Eaton, W. A. & Hofrichter, J. Laser temperature jump study of the helix⇔coil kinetics of an alanine peptide interpreted with a ‘kinetic zipper’ model. Biochemistry 36, 9200–9210 (1997)
Huang, G. S. & Oas, T. G. Submillisecond folding of monomeric lambda repressor. Proc. Natl Acad. Sci. USA 92, 6878–6882 (1995)
Matouschek, A., Kellis, J. T. Jr, Serrano, L. & Fersht, A. R. Mapping the transition state and pathway of protein folding by protein engineering. Nature 340, 122–126 (1989)
Baldwin, R. L. & Rose, G. D. Is protein folding hierarchic? II. Folding intermediates and transition states. Trends Biochem. Sci. 24, 77–83 (1999)
Karplus, M. & Weaver, D. L. Protein-folding dynamics—the diffusion–collision model and experimental-data. Prot. Sci. 3, 650–668 (1994)
Islam, S. A., Karplus, M. & Weaver, D. L. Application of the diffusion–collision model to the folding of three-helix bundle proteins. J. Mol. Biol. 318, 199–215 (2002)
Kippen, A. D., Arcus, V. L. & Fersht, A. R. Structural studies on peptides corresponding to mutants of the major alpha-helix of barnase. Biochemistry 33, 10013–10021 (1994)
Greenfield, N. & Fasman, G. D. Computed circular dichroism spectra for the evaluation of protein conformation. Biochemistry 8, 4108–4116 (1969)
Daggett, V. & Fersht, A. R. Is there a unifying mechanism of protein folding? Trends Biochem. Sci. 28, 19–26 (2003)
Hagen, S. J., Hofrichter, J., Szabo, A. & Eaton, W. A. Diffusion-limited contact formation in unfolded cytochrome c: estimating the maximum rate of protein folding. Proc. Natl Acad. Sci. USA 93, 11615–11617 (1996)
Bieri, O. et al. The speed limit for protein folding measured by triplet–triplet energy transfer. Proc. Natl Acad. Sci. USA 96, 9597–9601 (1999)
Cavanagh, J., Fairbrother, W. J., Palmer, A. G. & Skelton, N. J. Protein NMR Spectroscopy: Principles and Practice (Academic, San Diego, 1996)
Itzhaki, L. S., Neira, J. L. & Fersht, A. R. Hydrogen exchange in chymotrypsin inhibitor 2 probed by denaturants and temperature. J. Mol. Biol. 270, 89–98 (1997)
Bai, Y., Milne, J. S., Mayne, L. & Englander, S. W. Primary structure effects on peptide group hydrogen exchange. Proteins 17, 75–86 (1993)
Zhang, Y.-Z. Structural Biology and Molecular Biophysics. PhD thesis, Univ. Pennsylvania (1995)
Sandstrom, J. Dynamic NMR Spectroscopy (Academic, New York, 1982)
Grossmann, J. G., Hall, J. F., Kanbi, L. D. & Hasnain, S. S. The N-terminal extension of rusticyanin is not responsible for its acid stability. Biochemistry 41, 3613–3619 (2002)
Witty, M. et al. Structure of the periplasmic domain of Pseudomonas aeruginosa TolA: evidence for an evolutionary relationship with the TonB transporter protein. EMBO J. 21, 4207–4218 (2002)
Acknowledgements
This work was supported with an ‘Ikertzaileen prestakuntza’ grant (U.M.) from the Government of the Basque Country and with a Winston Churchill Scholarship (N.R.G). The computational studies were supported by the National Institutes of Health (to V.D.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Rights and permissions
About this article
Cite this article
Mayor, U., Guydosh, N., Johnson, C. et al. The complete folding pathway of a protein from nanoseconds to microseconds. Nature 421, 863–867 (2003). https://doi.org/10.1038/nature01428
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature01428
This article is cited by
-
Compliant mechanical response of the ultrafast folding protein EnHD under force
Communications Physics (2023)
-
Role of structural specificity of ZnO particles in preserving functionality of proteins in their corona
Scientific Reports (2021)
-
Ion–ion interactions in the denatured state contribute to the stabilization of CutA1 proteins
Scientific Reports (2018)
-
Cavity ring-up spectroscopy for ultrafast sensing with optical microresonators
Nature Communications (2015)
-
Structural dynamics of native and V260E mutant C-terminal domain of HIV-1 integrase
Journal of Computer-Aided Molecular Design (2015)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.