Abstract
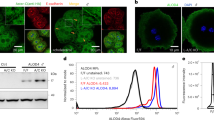
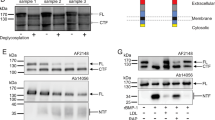
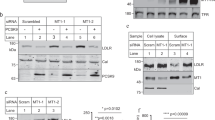
The effect of high-density lipoprotein (HDL) in protecting against atherosclerosis is usually attributed to its role in ‘reverse cholesterol transport’1. In this process, HDL particles mediate the efflux and the transport of cholesterol from peripheral cells to the liver for further metabolism and bile excretion. Thus, cell-surface receptors for HDL on hepatocytes are chief partners in the regulation of cholesterol homeostasis2. A high-affinity HDL receptor for apolipoprotein A-I (apoA-I) was previously identified on the surface of hepatocytes3,4. Here we show that this receptor is identical to the β-chain of ATP synthase, a principal protein complex of the mitochondrial inner membrane. Different experimental approaches confirm this ectopic localization of components of the ATP synthase complex and the presence of ATP hydrolase activity at the hepatocyte cell surface. Receptor stimulation by apoA-I triggers the endocytosis of holo-HDL particles (protein plus lipid) by a mechanism that depends strictly on the generation of ADP. We confirm this effect on endocytosis in perfused rat liver ex vivo by using a specific inhibitor of ATP synthase. Thus, membrane-bound ATP synthase has a previously unsuspected role in modulating the concentrations of extracellular ADP and is regulated by a principal plasma apolipoprotein.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Sviridov, D. & Nestel, P. Dynamics of reverse cholesterol transport: protection against atherosclerosis. Atherosclerosis 161, 245–254 (2002)
Fidge, N. H. High density lipoprotein receptors, binding proteins, and ligands. J. Lipid Res. 40, 187–201 (1999)
Barbaras, R., Collet, X., Chap, H. & Perret, B. Specific binding of free apolipoprotein A-I to a high-affinity binding site on HepG2 cells: characterization of two high-density lipoprotein sites. Biochemistry 33, 2335–2340 (1994)
Martinez, L. O. et al. Characterization of two high-density lipoprotein binding sites on porcine hepatocyte plasma membranes: contribution of scavenger receptor class B type I (SR-BI) to the low-affinity component. Biochemistry 39, 1076–1082 (2000)
Boyer, P. D. The ATP synthase: a splendid molecular machine. Annu. Rev. Biochem. 66, 717–749 (1997)
Stock, D., Leslie, A. G. & Walker, J. E. Molecular architecture of the rotary motor in ATP synthase. Science 286, 1700–1705 (1999)
Cabezon, E., Runswick, M. J., Leslie, A. G. & Walker, J. E. The structure of bovine IF(1), the regulatory subunit of mitochondrial F-ATPase. EMBO J. 20, 6990–6996 (2001)
Das, B., Mondragon, M. O., Sadeghian, M., Hatcher, V. B. & Norin, A. J. A novel ligand in lymphocyte-mediated cytotoxicity: expression of the beta subunit of H + transporting ATP synthase on the surface of tumour cell lines. J. Exp. Med. 180, 273–281 (1994)
Moser, T. L. et al. Angiostatin binds ATP synthase on the surface of human endothelial cells. Proc. Natl Acad. Sci. USA 96, 2811–2816 (1999)
Chang, S. Y., Park, S. G., Kim, S. & Kang, C. Y. Interaction of the C-terminal domain of p43 and the α subunit of ATP synthase: Its functional implication in endothelial cell proliferation. J. Biol. Chem. 277, 8388–8394 (2001)
Schippers, I. J. et al. Immortalized human hepatocytes as a tool for the study of hepatocytic (de-)differentiation. Cell Biol. Toxicol. 13, 375–86 (1997)
Williams, N., Amzel, L. M. & Pedersen, P. L. Proton ATPase of rat liver mitochondria: a rapid procedure for purification of a stable, reconstitutively active F1 preparation using a modified chloroform method. Anal. Biochem. 140, 581–588 (1984)
Moser, T. L. et al. Endothelial cell surface F1-F0 ATP synthase is active in ATP synthesis and is inhibited by angiostatin. Proc. Natl Acad. Sci. USA 98, 6656–6661 (2001)
Beisiegel, U. et al. Apolipoprotein E-binding proteins isolated from dog and human liver. Arteriosclerosis 8, 288–297 (1988)
Mahley, R. W., Hui, D. Y., Innerarity, T. L. & Beisiegel, U. Chylomicron remnant metabolism. Role of hepatic lipoprotein receptors in mediating uptake. Arteriosclerosis 9, I14–I18 (1989)
Ferrini, J. B., Pichard, L., Domergue, J. & Maurel, P. Long-term primary cultures of adult human hepatocytes. Chem. Biol. Interact. 107, 31–45 (1997)
Cabezon, E., Arechaga, I., Jonathan, P., Butler, G. & Walker, J. E. Dimerization of bovine F1-ATPase by binding the inhibitor protein, IF1 . J. Biol. Chem. 275, 28353–28355 (2000)
Cabezon, E., Butler, P. J., Runswick, M. J. & Walker, J. E. Modulation of the oligomerization state of the bovine F1-ATPase inhibitor protein, IF1, by pH. J. Biol. Chem. 275, 25460–25464 (2000)
Guendouzi, K., Collet, X., Perret, B., Chap, H. & Barbaras, R. Remnant high density lipoprotein2 particles produced by hepatic lipase display high-affinity binding and increased endocytosis into a human hepatoma cell line (HEPG2). Biochemistry 37, 14974–14980 (1998)
Acton, S. et al. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science 271, 518–520 (1996)
Silver, D. L., Nan, W., Xiao, X. & Tall, A. R. HDL particle uptake mediated by SR-BI results in selective sorting of HDL cholesterol from protein and polarized cholesterol secretion. J. Biol. Chem. 276, 25287–25293 (2001)
Walker, J. E. The regulation of catalysis in ATP synthase. Curr. Opin. Struct. Biol. 4, 912–918 (1994)
Wang, N., Silver, D. L., Thiele, C. & Tall, A. R. ATP-binding cassette transporter A1 (ABCA1) functions as a cholesterol efflux regulatory protein. J. Biol. Chem. 276, 23742–23747 (2001)
Bortnick, A. E. et al. The correlation of ABC1 mRNA levels with cholesterol efflux from various cell lines. J. Biol. Chem. 275, 28634–28640 (2000)
Burgess, J. W., Kiss, R. S., Zheng, H., Zachariah, S. & Marcel, Y. L. Trypsin-sensitive and lipid-containing sites of the macrophage extracellular matrix bind apolipoprotein A-I and participate in ABCA1-dependent cholesterol efflux. J. Biol. Chem. 277, 31318–31326 (2002)
Fitzgerald, M. L. et al. Naturally occurring mutations in ABCA1's largest extracellular loops can disrupt its direct interaction with apolipoprotein A-I. J. Biol. Chem. 277, 33178–33187 (2002)
Barrans, A. et al. Hepatic lipase induces the formation of pre-β1 high density lipoprotein (HDL) from triacylglycerol-rich HDL2 . J. Biol. Chem. 269, 11572–11577 (1994)
Johnsson, B., Lofas, S. & Lindquist, G. Immobilization of proteins to a carboxymethyldextran-modified gold surface for biospecific interaction analysis in surface plasmon resonance sensors. Anal. Biochem. 198, 268–277 (1991)
Sultan, C. et al. The novel inositol lipid phosphatidylinositol 3,4-bisphosphate is produced by human blood platelets upon thrombin stimulation. Biochem. J. 269, 831–834 (1990)
Acknowledgements
We thank G. Larrieu, for technical help, and P. Maurel INSERMU128, for the primary human hepatocytes.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Rights and permissions
About this article
Cite this article
Martinez, L., Jacquet, S., Esteve, JP. et al. Ectopic β-chain of ATP synthase is an apolipoprotein A-I receptor in hepatic HDL endocytosis. Nature 421, 75–79 (2003). https://doi.org/10.1038/nature01250
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature01250
This article is cited by
-
Ectopic ATP synthase stimulates the secretion of extracellular vesicles in cancer cells
Communications Biology (2023)
-
Spatial and temporal dynamics of ATP synthase from mitochondria toward the cell surface
Communications Biology (2023)
-
F1Fo adenosine triphosphate (ATP) synthase is a potential drug target in non-communicable diseases
Molecular Biology Reports (2023)
-
DJ-1 interacts with the ectopic ATP-synthase in endothelial cells during acute ischemia and reperfusion
Scientific Reports (2022)
-
MK2206 attenuates atherosclerosis by inhibiting lipid accumulation, cell migration, proliferation, and inflammation
Acta Pharmacologica Sinica (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.