Abstract
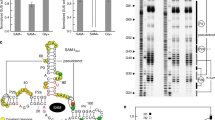
Although proteins fulfil most of the requirements that biology has for structural and functional components such as enzymes and receptors, RNA can also serve in these capacities. For example, RNA has sufficient structural plasticity to form ribozyme1,2 and receptor3,4 elements that exhibit considerable enzymatic power and binding specificity. Moreover, these activities can be combined to create allosteric ribozymes5,6 that are modulated by effector molecules. It has also been proposed7,8,9,10,11,12 that certain messenger RNAs might use allosteric mechanisms to mediate regulatory responses depending on specific metabolites. We report here that mRNAs encoding enzymes involved in thiamine (vitamin B1) biosynthesis in Escherichia coli can bind thiamine or its pyrophosphate derivative without the need for protein cofactors. The mRNA–effector complex adopts a distinct structure that sequesters the ribosome-binding site and leads to a reduction in gene expression. This metabolite-sensing regulatory system provides an example of a ‘riboswitch’ whose evolutionary origin might pre-date the emergence of proteins.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Cech, T. R. & Golden, B. L. The RNA World (eds Gesteland, R. F., Cech, T. R. & Atkins, J. F.) 321–350 (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, 1998)
Breaker, R. R. In vitro selection of catalytic polynucleotides. Chem. Rev. 97, 371–390 (1997)
Osborne, S. E. & Ellington, A. D. Nucleic acid selection and the challenge of combinatorial chemistry. Chem. Rev. 97, 349–370 (1997)
Hermann, T. & Patel, D. J. Adaptive recognition by nucleic acid aptamers. Science 287, 820–825 (2000)
Soukup, G. A. & Breaker, R. R. Engineering precision RNA molecular switches. Proc. Natl Acad. Sci. USA 96, 3584–3589 (1999)
Seetharaman, S., Zivarts, M., Sudarsan, N. & Breaker, R. R. Immobilized RNA switches for the analysis of complex chemical and biological mixtures. Nature Biotechnol. 19, 336–341 (2001)
Gold, L. et al. From oligonucleotide shapes to genomic SELEX: novel biological regulatory loops. Proc. Natl Acad. Sci. USA 94, 59–64 (1997)
Gold, L., Singer, B., He, Y. & Brody, E. SELEX and the evolution of genomes. Curr. Opin. Gen. Dev. 7, 848–851 (1997)
Nou, X. & Kadner, R. J. Adenosylcobalamin inhibits ribosome binding to btuB RNA. Proc. Natl Acad. Sci. USA 97, 7190–7195 (2000)
Gelfand, M. S., Mironov, A. A., Jomantas, J., Kozlov, Y. I. & Perumov, D. A. A conserved RNA structure element involved in the regulation of bacterial riboflavin synthesis genes. Trends Genet. 15, 439–442 (1999)
Miranda-Rios, J., Navarro, M. & Soberón, M. A conserved RNA structure (thi box) is involved in regulation of thiamin biosynthetic gene expression in bacteria. Proc. Natl Acad. Sci. USA 98, 9736–9741 (2001)
Stormo, G. D. & Ji, Y. Do mRNAs act as direct sensors of small molecules to control their expression? Proc. Natl Acad. Sci. USA 98, 9465–9467 (2001)
Webb, E. & Downs, D. Characterization of thiL, encoding thiamin-monophosphate kinase, in Salmonella typhimurium. J. Biol. Chem. 272, 15702–15707 (1997)
Nahvi, A. et al. Genetic control by a metabolite-binding mRNA. Chem. Biol. 9, 1043–1049 (2002)
Soukup, G. A. & Breaker, R. R. Relationship between internucleotide linkage geometry and the stability of RNA. RNA 5, 1308–1325 (1999)
Soukup, G. A., DeRose, E. E., Koizumi, M. & Breaker, R. R. Generating new ligand-binding RNAs by affinity maturation and disintegration of allosteric ribozymes. RNA 7, 524–536 (2001)
Zuker, M., Mathews, D. H. & Turner, D. H. RNA Biochemistry and Biotechnology (eds Barciszewski, J. & Clark, B. F. C.) 11–43 (NATO ASI Series, Kluwer Academic, Boston, 1999)
Mathews, D. H., Sabina, J., Zuker, M. & Turner, D. H. Expanded sequence dependence of thermodynamic parameters improves prediction of RNA secondary structure. J. Mol. Biol. 288, 911–940 (1999)
Gold, L., Poliski, B., Uhlenbeck, O. & Yarus, M. Diversity of oligonucleotide functions. Annu. Rev. Biochem. 64, 763–797 (1995)
Webb, E., Febres, F. & Downs, D. M. Thiamine pyrophosphate (TPP) negatively regulates transcription of some thi genes of Salmonella typhimurium. J. Bacteriol. 178, 2533–2538 (1996)
Gelfand, M. S., Mironov, A. A., Jomantas, J., Kozlov, Y. I. & Perumov, D. A. A conserved RNA structure element involved in the regulation of bacterial riboflavin synthesis genes. Trends Genet. 15, 439–442 (1999)
White, H. B. III Coenzymes as fossils of an earlier metabolic state. J. Mol. Evol. 7, 101–104 (1976)
White, H. B. III The Pyridine Nucleotide Coenzymes 1–17 (Academic, New York, 1982)
Benner, S. A., Ellington, A. D. & Tauer, A. Modern metabolism as a palimpsest of the RNA world. Proc. Natl Acad. Sci. USA 86, 7054–7058 (1989)
Joyce, G. F. The antiquity of RNA-based evolution. Nature 418, 214–221 (2002)
Vander Horn, P. B., Backstrom, A. D., Stewart, V. & Begley, T. P. Structural genes for thiamine biosynthetic enzymes (thiCEFGH) in Escherichia coli K-12. J. Bacteriol. 175, 982–992 (1993)
Simons, R. W., Houman, F. & Kleckner, N. Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene 53, 85–96 (1987)
Miller, J. H. A Short Course in Bacterial Genetics 72 (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York, 1992)
Acknowledgements
We thank members of the Breaker laboratory for comments on the manuscript, especially N. Sudarsan for discussions. This work was supported by the NIH and the NSF, and by a fellowship to R.R.B. from the David and Lucile Packard Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Rights and permissions
About this article
Cite this article
Winkler, W., Nahvi, A. & Breaker, R. Thiamine derivatives bind messenger RNAs directly to regulate bacterial gene expression. Nature 419, 952–956 (2002). https://doi.org/10.1038/nature01145
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature01145
This article is cited by
-
Exploring l-isoleucine riboswitches for enhancing 4-hydroxyisoleucine production in Corynebacterium glutamicum
Biotechnology Letters (2023)
-
The Application Potential of Synthetic Biology in Microbial Communication
Current Clinical Microbiology Reports (2023)
-
Programming inactive RNA-binding small molecules into bioactive degraders
Nature (2023)
-
Development and characterization of a glycine biosensor system for fine-tuned metabolic regulation in Escherichia coli
Microbial Cell Factories (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.