Abstract
Pressure to ban the hunting of foxes with hounds in Britain has fuelled debate about its contribution to the control of fox populations. We took advantage of a nationwide one-year ban on fox-hunting during the outbreak of foot-and-mouth disease (FMD) in 2001 to examine this issue and found that the ban had no measurable impact on fox numbers in randomly selected areas. Our results argue against suggestions that fox populations would increase markedly in the event of a permanent ban on hunting.
Similar content being viewed by others
Main
In the 2000–2001 season, foxes were hunted (Fig. 1) by 196 registered foxhound packs and 7 harrier packs1, and by an unknown number of unregistered packs, the latter mainly in southwest England and Wales2. However, their contribution to controlling fox numbers is unclear. The arguments are that the number of foxes killed by hunting is relatively small, and therefore that hunting has little influence in regulating population size3, and conversely that hunting is important for limiting fox numbers, particularly in combination with other culling practices, so a ban would lead to a significant population increase with consequential economic damage and loss of biodiversity4.
The main fox-hunting season in Britain runs from November to March/April; cub-hunting lasts from August to October, and 'problem' foxes are targeted after the main season. From 23 February to 17 December 2001, the Foot-and-Mouth Disease Declaratory (Controlled Area) Order 2001 banned hunting. From 17 December, some hunts were licensed to hunt in FMD-free areas, and from 11 February 2002, any hunt could be licensed if certain restrictions were observed. Hunting was thus banned for ten months and severely curtailed for two further months.
With 240,000 adult foxes, and 425,000 cubs born in Britain each year5, 64% mortality per annum is required to prevent growth of the fox population6. A one-year ban should therefore lead to a detectable population increase if hunting has a significant impact on fox numbers.
To quantify the changes in fox numbers during the hunting ban, we surveyed 160 one-kilometre squares between 1 February and 17 March in 1999 and 2000 (pre-FMD) and 2002 (post-FMD) — that is, towards the end of the main culling period and just before the cubs are born. We estimated relative fox density by using faecal-accumulation rates7 along transects (mean length, 6.9 km) that followed linear features; we surveyed each transect twice in each period (pre- and post-FMD). We removed all fox faeces on the first visit, and counted fresh faeces 2–6 weeks later. We calculated faecal density for each transect in each period (F) as F = S/KD, where S is the number of faeces on the second visit, K is the transect length in kilometres, and D is the number of days between visits. Absolute changes in faecal density were calculated as Fpost-FMD − Fpre-FMD, and relative changes (R′) as log(R + 1), where R = (Fpost-FMD − Fpre-FMD)/(Fpre-FMD + 1).
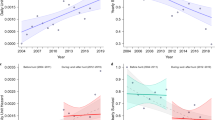
Overall, faecal density declined by 4.7% (Fig. 2a), with no significant difference in the number of squares where faecal density increased (n = 83) compared with those that decreased or remained unchanged (n = 77) in 'hunted' (n = 118) as oppposed to 'not-hunted' (n = 42) squares (χ21 = 0.19, P = 0.66). Moreover, neither the absolute (t158 = 0.60, P = 0.55) nor the relative (t158 = 0.51, P = 0.61) change in faecal density differed between 'hunted' and 'not-hunted' squares. 'Hunted' squares lay within the area covered by a hunt, as designated on a map issued in 1996 (ref. 8).
a, Mean (±s.d.) change in absolute faecal density by region (post-FMD minus pre-FMD). b, Relationship between regional reduction in hunting pressure and relative change in faecal density. CE, central England, n = 16; EE, eastern England, n = 23; M, Midlands, n = 21; NE, northern England, n = 15; NS, northern Scotland, n = 8; SE, southern England, n = 38; SS, southern Scotland, n = 9; SWE, southwest England, n = 25; W, Wales, n = 5. The results of this survey are posted by The Mammal Society at http://www.mammal.org.uk.
R′ values were normally distributed (Kolmogorov–Smirnov goodness-of-fit test, Z = 0.71, P = 0.70), with homogenous variances between regions (Levene statistic, F8,151 = 1.84, P = 0.07). There was a significant difference between nine regions in the relative magnitude of the change in faecal density (ANOVA, F8,145 = 2.69, P= 0.01). Tukey's HSD post hoc comparisons showed a significant increase in eastern England (one sample t-test, t22 = 2.52, P = 0.02) and a significant decrease in southern England (t37 = − 2.65, P = 0.01). No other region deviated significantly from zero in test values.
We evaluated the relative change in faecal density against a regional index of hunting pressure (number of days hunted by registered packs per week per km2 in the 2000–2001 season1). There was no association between the reduction in hunting pressure in each region and the relative change in faecal density (linear regression with replication, r2 = 0.007, F1,158 = 1.18, P = 0.28; Fig. 2b). We conclude that there was no significant change in fox numbers during the one-year hunting ban, and that in most regions the average faecal density had declined. Our results therefore support the view taken by the Committee of Inquiry into Hunting with Dogs2 that a permanent ban on hunting is unlikely to result in a dramatic increase in fox numbers.
References
Anon. Baily's Hunting Directory 2000–2001 (Pearson, Cambridge, 2000).
Burns, L., Edwards, V., Marsh, J., Soulsby, L. & Winter, M. Report of the Committee of Inquiry into Hunting with Dogs in England and Wales (Stationery Office, London, 2000).
Macdonald, D. W. & Johnson, P. J. in The Exploitation of Mammal Populations (eds Taylor, V. J. & Dunstone, N.) 160–207 (Chapman & Hall, London, 1996).
Tapper, S. A Question of Balance: Game Animals and their Role in the British Countryside (Game Conservancy Trust, Fordingbridge, Hampshire, 1999).
Harris, S., Morris, P., Wray, S. & Yalden, D. A Review of British Mammals: Population Estimates and Conservation Status of British Mammals other than Cetaceans (Joint Nat. Conserv. Commit., Peterborough, 1995).
Harris, S. & Saunders, G. Symp. Zool. Soc. Lond. 65, 441–464 (1993).
Putman, R. J. Mammal Rev. 14, 79–97 (1984).
Alexander, K. Baily's Hunting Directory 1996–1997 (Pearson, Cambridge, 1996).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
financial, declared none; P.J.B. and C.C.W. were funded by the International Fund for Animal Welfare.
Rights and permissions
About this article
Cite this article
Baker, P., Harris, S. & Webbon, C. Effect of British hunting ban on fox numbers. Nature 419, 34 (2002). https://doi.org/10.1038/419034a
Issue Date:
DOI: https://doi.org/10.1038/419034a
This article is cited by
-
No evidence for spatial variation in predation risk following restricted-area fox culling
BMC Ecology (2019)
-
A prickly problem: developing a volunteer-friendly tool for monitoring populations of a terrestrial urban mammal, the West European hedgehog (Erinaceus europaeus)
Urban Ecosystems (2018)
-
The value of a random sampling design for annual monitoring of national populations of larger British terrestrial mammals
European Journal of Wildlife Research (2014)
-
Reproduction of the red fox Vulpes vulpes in western France: does staining improve estimation of litter size from placental scar counts?
European Journal of Wildlife Research (2011)
-
Surveying carnivores at large spatial scales: a comparison of four broad-applied methods
Biodiversity and Conservation (2007)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.