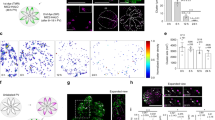
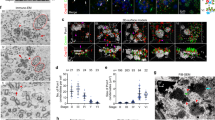
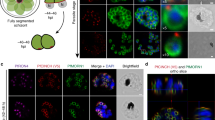
Abstract
Two models have been put forward to explain the growth of new Golgi during the cell cycle. The first suggests that a new Golgi grows out of the endoplasmic reticulum by de novo synthesis1. The second suggests that a pre-existing Golgi is needed for the growth of a new one, that is, the Golgi is an autonomously replicating organelle2. To resolve this issue, we have exploited the simplicity of the apicomplexan parasite Toxoplasma gondii3, which has only a single Golgi stack4. Here we show, by using video fluorescence microscopy and three-dimensional reconstructions of serial thin sections, that the Golgi grows by a process of lateral extension followed by medial fission. Further fission leads to the inheritance by each daughter of a pair of Golgi structures, which then coalesce to re-form a single Golgi. Our results indicate that new Golgi grow by autonomous duplication and raise the possibility that the Golgi is a paired structure that is analogous to centrioles5.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Lippincott-Schwartz, J. & Zaal, K. J. Cell cycle maintenance and biogenesis of the Golgi complex. Histochem. Cell. Biol. 114, 93–103 (2000)
Bracker, C. E., Morre, D. J. & Grove, S. N. Structure, differentiation, and multiplication of Golgi-apparatus in fungal hyphae. Protoplasma 194, 250–274 (1996)
Joiner, K. A. & Roos, D. S. Secretory traffic in the eukaryotic parasite Toxoplasma gondii: less is more. J. Cell Biol. 157, 557–563 (2002)
Roos, D. S. et al. Transport and trafficking: Toxoplasma as a model for Plasmodium. Novartis Found. Symp. 226, 176–195 (1999)
Marshall, W. Centrioles take center stage. Curr. Biol. 11, R487–R496 (2001)
Kelly, T. J. & Brown, G. W. Regulation of chromosome replication. Annu. Rev. Biochem. 69, 829–880 (2000)
Warren, G. Membrane partitioning during cell division. Annu. Rev. Biochem. 62, 323–348 (1993)
Whaley, W. G. The Golgi Apparatus (Springer, New York, 1975)
Preuss, D., Mulholland, J., Franzusoff, A., Segev, N. & Botstein, D. Characterization of the Saccharomyces Golgi complex through the cell cycle by immunoelectron microscopy. Mol. Biol. Cell 3, 789–803 (1992)
Zaal, K. J. et al. Golgi membranes are absorbed into and reemerge from the ER during mitosis. Cell 99, 589–601 (1999)
Presley, J. F. et al. Golgi membrane dynamics. Mol. Biol. Cell 9, 1617–1626 (1998)
Pelletier, L., Jokitalo, E. & Warren, G. The effect of Golgi depletion on exocytic transport. Nature Cell Biol. 2, 840–846 (2000)
Shima, D. T., Haldar, K., Pepperkok, R., Watson, R. & Warren, G. Partitioning of the Golgi apparatus during mitosis in living HeLa cells. J. Cell Biol. 137, 1211–1228 (1997)
Cole, N. B., Sciaky, N., Marotta, A., Song, J. & Lippincott-Schwartz, J. Golgi dispersal during microtubule disruption—regeneration of Golgi stacks at peripheral endoplasmic-reticulum exit sites. Mol. Biol. Cell 7, 631–650 (1996)
Wooding, S. & Pelham, H. R. The dynamics of Golgi protein traffic visualized in living yeast cells. Mol. Biol. Cell 9, 2667–2680 (1998)
Rossanese, O. W. et al. Golgi structure correlates with transitional endoplasmic reticulum organization in Pichia pastoris and Saccharomyces cerevisiae. J. Cell Biol. 145, 69–81 (1999)
Dubremetz, J. F., Garcia-Reguet, N., Conseil, V. & Fourmaux, M. N. Apical organelles and host-cell invasion by Apicomplexa. Int. J. Parasitol. 28, 1007–1013 (1998)
Hager, K. M., Striepen, B., Tilney, L. G. & Roos, D. S. The nuclear envelope serves as an intermediary between the ER and Golgi complex in the intracellular parasite Toxoplasma gondii. J. Cell Sci. 112, 2631–2638 (1999)
Sheffield, H. G. & Melton, M. L. The fine structure and reproduction of Toxoplasma gondii. J. Parasitol. 54, 209–226 (1968)
Radke, J. R. et al. Defining the cell cycle for the tachyzoite stage of Toxoplasma gondii. Mol. Biochem. Parasitol. 115, 165–175 (2001)
Hu, K. et al. Daughter cell assembly in the protozoan parasite Toxoplasma gondii. Mol. Biol. Cell 13, 593–606 (2002)
Seemann, J., Jokitalo, E., Pypaert, M. & Warren, G. Matrix proteins can generate the higher order architecture of the Golgi apparatus. Nature 407, 1022–1026 (2000)
Ward, T. H., Polishchuk, R. S., Caplan, S., Hirschberg, K. & Lippincott-Schwartz, J. Maintenance of Golgi structure and function depends on the integrity of ER export. J. Cell Biol. 155, 557–570 (2001)
Wiggins, C. A. R. & Munro, S. Activity of the yeast mnn1 α-1,3-mannosyltransferase requires a motif conserved in many other families of glycosyltransferases. Proc. Natl Acad. Sci. USA 95, 7945–7950 (1998)
Odenthal-Schnittler, M., Tomavo, S., Becker, D., Dubremetz, J. F. & Schwarz, R. T. Evidence for N-linked glycosylation in Toxoplasma gondii. Biochem. J. 291, 713–721 (1993)
Lujan, H. D. et al. Developmental induction of Golgi structure and function in the primitive eukaryote Giardia lamblia. J. Biol. Chem. 270, 4612–4618 (1995)
Field, H., Sherwin, T., Smith, A. C., Gull, K. & Field, M. C. Cell-cycle and developmental regulation of TbRAB31 localisation, a GTP- locked Rab protein from Trypanosoma brucei. Mol. Biochem. Parasitol. 106, 21–35 (2000); erratum 107, 329–330 (2000)
Piel, M., Nordberg, J., Euteneuer, U. & Bornens, M. Centrosome-dependent exit of cytokinesis in animal cells. Science 291, 1550–1553 (2001)
Roos, D. S., Donald, R. G. K., Morrissette, N. S. & Moulton, A. L. C. Molecular tools for genetic dissection of the protozoan parasite Toxoplasma gondii. Methods Cell Biol. 45, 27–63 (1994)
Acknowledgements
We thank F. Barr and T. Nilsson for the GRASP55 and NAGTI plasmids; S. Trombetta for recombinant GFP; the Joiner and Roos labs—mainly F. Quittnat, T. Stedman and O. Harb—for advice and discussion; N. Andrews for the use of her cell-culture room; C. Roy and J. Kagan for help with confocal microscopy; K. Zichichi, C. Horensavitz and C. Deloyer-Pypaert for help with electron microscopy; Olympus for providing some of the microscopes; J. Shorter and E. Procyk for critically reading the manuscript; and the Warren and Mellman labs for discussions. This work was supported by National Institutes of Health grants (to G.W., I.M., K.A.J. and D.S.R.) and the Ludwig Institute for Cancer Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Rights and permissions
About this article
Cite this article
Pelletier, L., Stern, C., Pypaert, M. et al. Golgi biogenesis in Toxoplasma gondii. Nature 418, 548–552 (2002). https://doi.org/10.1038/nature00946
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature00946
This article is cited by
-
The life-cycle of Toxoplasma gondii reviewed using animations
Parasites & Vectors (2020)
-
A genome-wide analysis of coatomer protein (COP) subunits of apicomplexan parasites and their evolutionary relationships
BMC Genomics (2019)
-
A highly dynamic F-actin network regulates transport and recycling of micronemes in Toxoplasma gondii vacuoles
Nature Communications (2019)
-
High Throughput Analysis of Golgi Structure by Imaging Flow Cytometry
Scientific Reports (2017)
-
Oxidative stress generated during monensin treatment contributes to altered Toxoplasma gondii mitochondrial function
Scientific Reports (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.