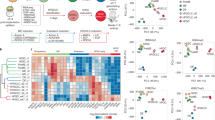
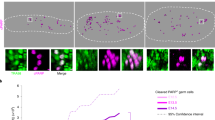
Abstract
Germ cell fate in mice is induced in proximal epiblast cells by the extra-embryonic ectoderm, and is not acquired through the inheritance of any preformed germ plasm. To determine precisely how germ cells are specified, we performed a genetic screen between single nascent germ cells and their somatic neighbours that share common ancestry. Here we show that fragilis, an interferon-inducible transmembrane protein, marks the onset of germ cell competence, and we propose that through homotypic association, it demarcates germ cells from somatic neighbours. Using single-cell gene expression profiles, we also show that only those cells with the highest expression of fragilis subsequently express stella, a gene that we detected exclusively in lineage-restricted germ cells. The stella positive nascent germ cells exhibit repression of homeobox genes, which may explain their escape from a somatic cell fate and the retention of pluripotency.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Weismann, A. Das Keimplasma. Eine Theorie der Vererbung (Gustav Fischer, Jena, 1892)
Eddy, E. M. Germ plasm and the differentiation of the germ cell line. Int. Rev. Cytol. 43, 229–280 (1975)
Seydoux, G. & Strome, S. Launching the germline in Caenorhabditis elegans: regulation of gene expression in early germ cells. Development 126, 3275–3283 (1999)
Wylie, C. Germ cells. Cell 96, 165–174 (1999)
Lawson, K. A. & Hage, W. J. Clonal analysis of the origin of primordial germ cells in the mouse. Ciba Found. Symp. 182, 68–84 (1994)
Lawson, K. A. et al. Bmp4 is required for the generation of primordial germ cells in the mouse embryo. Genes Dev. 13, 424–436 (1999)
McLaren, A. Signaling for germ cells. Genes Dev. 13, 373–376 (1999)
Tam, P. P. & Zhou, S. X. The allocation of epiblast cells to ectodermal and germ-line lineages is influenced by the position of the cells in the gastrulating mouse embryo. Dev. Biol. 178, 124–132 (1996)
Yoshimizu, T., Obinata, M. & Matsui, Y. Stage-specific tissue and cell interactions play key roles in mouse germ cell specification. Development 128, 481–490 (2001)
Ying, Y., Liu, X. M., Marble, A., Lawson, K. A. & Zhao, G. Q. Requirement of Bmp8b for the generation of primordial germ cells in the mouse. Mol. Endocrinol. 14, 1053–1063 (2000)
Ying, Y., Qi, X. & Zhao, G. Q. Induction of primordial germ cells from murine epiblasts by synergistic action of BMP4 and BMP8B signaling pathways. Proc. Natl Acad. Sci. USA 98, 7858–7862 (2001)
Ying, Y. & Zhao, G. Q. Cooperation of endoderm-derived BMP2 and extraembryonic ectoderm-derived BMP4 in primordial germ cell generation in the mouse. Dev. Biol. 232, 484–492 (2001)
Chiquoine, A. D. The identification, origin and migration of the primordial germ cells in the mouse embryo. Anat. Rec. 118, 135–146 (1954)
Ginsburg, M., Snow, M. H. & McLaren, A. Primordial germ cells in the mouse embryo during gastrulation. Development 110, 521–528 (1990)
MacGregor, G. R., Zambrowicz, B. P. & Soriano, P. Tissue non-specific alkaline phosphatase is expressed in both embryonic and extraembryonic lineages during mouse embryogenesis but is not required for migration of primordial germ cells. Development 121, 1487–1496 (1995)
Nichols, J. et al. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell 95, 379–391 (1998)
Pesce, M., Gross, M. K. & Scholer, H. R. In line with our ancestors: Oct-4 and the mammalian germ. Bioessays 20, 722–732 (1998)
Yeom, Y. I. et al. Germline regulatory element of Oct-4 specific for the totipotent cycle of embryonal cells. Development 122, 881–894 (1996)
Downs, K. M. & Davies, T. Staging of gastrulating mouse embryos by morphological landmarks in the dissecting microscope. Development 118, 1255–1266 (1993)
Brady, G. & Iscove, N. N. Construction of cDNA libraries from single cells. Methods Enzymol. 225, 611–623 (1993)
Dulac, C. & Axel, R. A novel family of genes encoding putative pheromone receptors in mammals. Cell 83, 195–206 (1995)
Tanabe, Y., William, C. & Jessell, T. M. Specification of motor neuron identity by the MNR2 homeodomain protein. Cell 95, 67–80 (1998)
Frohman, M. A., Boyle, M. & Martin, G. R. Isolation of the mouse Hox-2.9 gene; analysis of embryonic expression suggests that positional information along the anterior-posterior axis is specified by mesoderm. Development 110, 589–607 (1990)
Deblandre, G. A. et al. Expression cloning of an interferon-inducible 17-kDa membrane protein implicated in the control of cell growth. J. Biol. Chem. 270, 23860–23866 (1995)
Friedman, R. L., Manly, S. P., McMahon, M., Kerr, I. M. & Stark, G. R. Transcriptional and posttranscriptional regulation of interferon-induced gene expression in human cells. Cell 38, 745–755 (1984)
Evans, S. S., Collea, R. P., Leasure, J. A. & Lee, D. B. IFN-α induces homotypic adhesion and Leu-13 expression in human B lymphoid cells. J. Immunol. 150, 736–747 (1993)
Evans, S. S., Lee, D. B., Han, T., Tomasi, T. B. & Evans, R. L. Monoclonal antibody to the interferon-inducible protein Leu-13 triggers aggregation and inhibits proliferation of leukemic B cells. Blood 76, 2583–2593 (1990)
Aravind, L. & Koonin, E. V. SAP—a putative DNA-binding motif involved in chromosomal organization. Trends Biochem. Sci. 25, 112–114 (2000)
Crossley, P. H. & Martin, G. R. The mouse Fgf8 gene encodes a family of polypeptides and is expressed in regions that direct outgrowth and patterning in the developing embryo. Development 121, 439–451 (1995)
Herrmann, B. G. Expression pattern of the Brachyury gene in whole-mount TWis/TWis mutant embryos. Development 113, 913–917 (1991)
Herrmann, B. G., Labeit, S., Poustka, A., King, T. R. & Lehrach, H. Cloning of the T gene required in mesoderm formation in the mouse. Nature 343, 617–622 (1990)
Barnes, J. D., Crosby, J. L., Jones, C. M., Wright, C. V. & Hogan, B. L. Embryonic expression of Lim-1, the mouse homolog of Xenopus Xlim-1, suggests a role in lateral mesoderm differentiation and neurogenesis. Dev. Biol. 161, 168–178 (1994)
Bastian, H. & Gruss, P. A murine even-skipped homologue, Evx 1, is expressed during early embryogenesis and neurogenesis in a biphasic manner. EMBO J. 9, 1839–1852 (1990)
Fujii, T. et al. Expression patterns of the murine LIM class homeobox gene lim1 in the developing brain and excretory system. Dev. Dyn. 199, 73–83 (1994)
Kuramochi-Miyagawa, S. et al. Two mouse piwi-related genes: miwi and mili. Mech. Dev. 108, 121–133 (2001)
Fujiwara, Y. et al. Isolation of a DEAD-family protein gene that encodes a murine homolog of Drosophila vasa and its specific expression in germ cell lineage. Proc. Natl Acad. Sci. USA 91, 12258–12262 (1994)
Nieuwkoop, P. D. & Satasurya, L. A. Primordial Germ Cells in the Chordates (Cambridge Univ. Press, Cambridge, 1979)
Johnson, A. D., Bachvarova, R. F., Drum, M. & Masi, T. Expression of axolotl dazl RNA, a marker of germ plasm: widespread maternal RNA and onset of expression in germ cells approaching the gonad. Dev. Biol. 234, 402–415 (2001)
Johnson, A. D., Bachvarova, R. F., Masi, T. & Drum, M. in Germ Cells: Cold Spring Harbor Lab. Meeting 61 (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, 2000)
Toyooka, Y. et al. Expression and intracellular localization of mouse Vasa-homologue protein during germ cell development. Mech. Dev. 93, 139–149 (2000)
Gurdon, J. B., Kato, K. & Lemaire, P. The community effect, dorsalization and mesoderm induction. Curr. Opin. Genet. Dev. 3, 662–667 (1993)
Reid, L. E. et al. A single DNA response element can confer inducibility by both α- and γ-interferons. Proc. Natl Acad. Sci. USA 86, 840–844 (1989)
Barlow, D. P., Randle, B. J. & Burke, D. C. Interferon synthesis in the early post-implantation mouse embryo. Differentiation 27, 229–235 (1984)
Kita, M. et al. Expression of cytokines and interferon-related genes in the mouse embryo. C.R. Seances Soc. Biol. Fil. 188, 593–600 (1994)
Gomperts, M., Garcia-Castro, M., Wylie, C. & Heasman, J. Interactions between primordial germ cells play a role in their migration in mouse embryos. Development 120, 135–141 (1994)
Sato, M. et al. Identification of PGC7, a new gene expressed specifically in preimplantation embryos and germ cells. Mech. Dev. 113, 91–94 (2002)
Saitou, M. et al. Occludin-deficient embryonic stem cells can differentiate into polarized epithelial cells bearing tight junctions. J. Cell Biol. 141, 397–408 (1998)
Henrique, D. et al. Expression of a Delta homologue in prospective neurons in the chick. Nature 375, 787–790 (1995)
Wilkinson, D. G. & Nieto, M. A. Detection of messenger RNA by in situ hybridization to tissue sections and whole mounts. Methods Enzymol. 225, 361–373 (1993)
Winnier, G., Blessing, M., Labosky, P. A. & Hogan, B. L. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev. 9, 2105–2116 (1995)
Acknowledgements
We thank K. Lawson for providing unpublished information and for discussions, and A. McLaren for critical comments during the course of this study. We also thank Y. Tanabe and T. Yamamoto for helpful information concerning the construction of single cell cDNAs. We are grateful to the Wellcome Trust for support through a Wellcome Travelling Fellowship to M.S. and for a Programme Grant to M.A.S.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Rights and permissions
About this article
Cite this article
Saitou, M., Barton, S. & Surani, M. A molecular programme for the specification of germ cell fate in mice. Nature 418, 293–300 (2002). https://doi.org/10.1038/nature00927
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature00927
This article is cited by
-
Well-TEMP-seq as a microwell-based strategy for massively parallel profiling of single-cell temporal RNA dynamics
Nature Communications (2023)
-
Dppa3 Improves the Germline Competence of Pluripotent Stem Cells
Stem Cell Reviews and Reports (2023)
-
Germline stem cells in human
Signal Transduction and Targeted Therapy (2022)
-
Interplay between chromatin marks in development and disease
Nature Reviews Genetics (2022)
-
The role of m6A modification in the biological functions and diseases
Signal Transduction and Targeted Therapy (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.