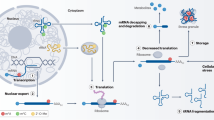
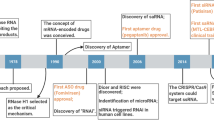
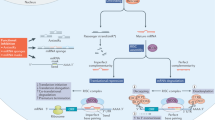
Abstract
RNA is a versatile biological macromolecule that is crucial in mobilizing and interpreting our genetic information. It is not surprising then that researchers have sought to exploit the inherent properties of RNAs so as to interfere with or repair dysfunctional nucleic acids or proteins and to stimulate the production of therapeutic gene products in a variety of pathological situations. The first generation of the resulting RNA therapeutics are now being evaluated in clinical trials, raising significant interest in this emerging area of medical research.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Green, P. J., Pines, O. & Inouye, M. The role of antisense RNA in gene regulation. Annu. Rev. Biochem. 55, 569–597 (1986).
Pestka, S., Daugherty, B. L., Jung, V., Hotta, K. & Pestka, R. K. Anti-mRNA: specific inhibition of translation of single mRNA molecules. Proc. Natl Acad. Sci. USA 81, 7525–7528 (1984).
Coleman, J., Green, P. J. & Inouye, M. The use of RNAs complementary to specific mRNAs to regulate the expression of individual bacterial genes. Cell 37, 429–436 (1984).
Izant, J. G. & Weintraub, H. Constitutive and conditional suppression of exogenous and endogenous genes by anti-sense RNA. Science 229, 345–352 (1985).
van der Krol, A. R., Mol, J. N. & Stuitje, A. R. Modulation of eukaryotic gene expression by complementary RNA or DNA sequences. Biotechniques 6, 958–976 (1988).
Sullenger, B. A., Lee, T. C., Smith, C. A., Ungers, G. E. & Gilboa, E. Expression of chimeric tRNA-driven antisense transcripts renders NIH 3T3 cells highly resistant to Moloney murine leukemia virus replication. Mol. Cell. Biol. 10, 6512–6523 (1990).
Kruger, K. et al. Self-splicing RNA: autoexcision and autocyclization of the ribosomal RNA intervening sequence of Tetrahymena. Cell 31, 147–157 (1982).
Guerrier-Takada, C., Gardiner, K., Marsh, T., Pace, N. & Altman, S. The RNA moiety of ribonuclease P is the catalytic subunit of the enzyme. Cell 35, 849–857 (1983).
Uhlenbeck, O. C. A small catalytic oligoribonucleotide. Nature 328, 596–600 (1987).
Haseloff, J. & Gerlach, W. L. Simple RNA enzymes with new and highly specific endoribonuclease activities. Nature 334, 585–591 (1988).
Cech, T. R. Ribozymes and their medical implications. J. Am. Med. Assoc. 260, 3030–3034 (1988).
Usman, N. & Blatt, L. M. Nuclease-resistant synthetic ribozymes: developing a new class of therapeutics. J. Clin. Invest. 106, 1197–1202 (2000).
Symons, R. H. Small catalytic RNAs. Annu. Rev. Biochem. 61, 641–671 (1992).
Bauer, G. et al. Inhibition of human immunodeficiency virus-1 (HIV-1) replication after transduction of granulocyte colony-stimulating factor-mobilized CD34+ cells from HIV-1-infected donors using retroviral vectors containing anti-HIV-1 genes. Blood 89, 2259–2267 (1997).
Wong-Staal, F., Poeschla, E. M. & Looney, D. J. A controlled, Phase 1 clinical trial to evaluate the safety and effects in HIV-1 infected humans of autologous lymphocytes transduced with a ribozyme that cleaves HIV-1 RNA. Hum. Gene Ther. 9, 2407–2425 (1998).
Amado, R. G. et al. A phase I trial of autologous CD34+ hematopoietic progenitor cells transduced with an anti-HIV ribozyme. Hum. Gene Ther. 10, 2255–2270 (1999).
Sullenger, B. A. & Cech, T. R. Tethering ribozymes to a retroviral packaging signal for destruction of viral RNA. Science 262, 1566–1569 (1993).
Lee, N. S., Bertrand, E. & Rossi, J. mRNA localization signals can enhance the intracellular effectiveness of hammerhead ribozymes. RNA 5, 1200–1209 (1999).
Beigelman, L. et al. Chemical modification of hammerhead ribozymes. Catalytic activity and nuclease resistance. J. Biol. Chem. 270, 25702–25708 (1995).
Wincott, F. et al. Synthesis, deprotection, analysis and purification of RNA and ribozymes. Nucleic Acids Res. 23, 2677–2684 (1995).
Pavco, P. A. et al. Antitumor and antimetastatic activity of ribozymes targeting the messenger RNA of vascular endothelial growth factor receptors. Clin. Cancer Res. 6, 2094–2103 (2000).
Macejak, D. G. et al. Inhibition of hepatitis C virus (HCV)-RNA-dependent translation and replication of a chimeric HCV poliovirus using synthetic stabilized ribozymes. Hepatology 31, 769–776 (2000).
Elbashir, S. M. et al. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 411, 494–498 (2001).
Sui, G. et al. A DNA vector-based RNAi technology to suppress gene expression in mammalian cells. Proc. Natl Acad. Sci. USA 99, 5515–5520 (2002).
Miyagishi, M. & Taira, K. U6 promoter-driven siRNA with four uridine 3′ overhangs efficiently suppress targeted gene expression in mammalian cells. Nature Biotechnol. 20, 497–500 (2002).
Lee, N. S. et al. Expression of small interfering RNAs targeted against HIV-1 rev transcripts in human cells. Nature Biotechnol. 20, 500–505 (2002).
Paul, C. P. et al. Effective expression of small interfering RNA in human cells. Nature Biotechnol. 20, 505–508 (2002).
Guo, H. et al. Group II introns designed to insert into therapeutically relevant DNA target sites in human cells. Science 289, 452–457 (2000).
Sullenger, B. A. & Cech, T. R. Ribozyme-mediated repair of defective mRNA by targeted, trans-splicing. Nature 371, 619–622 (1994).
Jones, J. T., Lee, S. W. & Sullenger, B. A. Tagging ribozyme reaction sites to follow trans-splicing in mammalian cells. Nature Med. 2, 643–648 (1996).
Phylactou, L. A., Darrah, C. & Wood, M. J. Ribozyme-mediated trans-splicing of a trinucleotide repeat. Nature Genet. 18, 378–381 (1998).
Watanabe, T. & Sullenger, B. A. Induction of wild-type p53 activity in human cancer cells by ribozymes that repair mutant p53 transcripts. Proc. Natl Acad. Sci. USA 97, 8490–8494 (2000).
Lan, N., Howrey, R. P., Lee, S. W., Smith, C. A. & Sullenger, B. A. Ribozyme-mediated repair of sickle beta-globin mRNAs in erythrocyte precursors. Science 280, 1593–1596 (1998).
Puttaraju, M., Jamison, S. F., Mansfield, S. G., Garcia-Blanco, M. A. & Mitchell, L. G. Spliceosome-mediated RNA trans-splicing as a tool for gene therapy. Nature Biotechnol. 17, 246–252 (1999).
Puttaraju, M., DiPasquale, J., Baker, C. C., Mitchell, L. G. & Garcia-Blanco, M. A. Messenger RNA repair and restoration of protein function by spliceosome-mediated RNA trans-splicing. Mol. Ther. 4, 105–114 (2001).
Liu, X. et al. Partial correction of endogenous ΔF508 CFTR in human cystic fibrosis airway epithelia by spliceosome-mediated RNA trans-splicing. Nature Biotechnol. 20, 47–52 (2002).
Kikumori, T., Cote, G. J. & Gagel, R. F. Promiscuity of pre-mRNA spliceosome-mediated trans splicing: a problem for gene therapy? Hum. Gene Ther. 12, 1429–1441 (2001).
Kohler, U., Ayre, B. G., Goodman, H. M. & Haseloff, J. Trans-splicing ribozymes for targeted gene delivery. J. Mol. Biol. 285, 1935–1950 (1999).
Ayre, B. G., Kohler, U., Goodman, H. M. & Haseloff, J. Design of highly specific cytotoxins by using trans-splicing ribozymes. Proc. Natl Acad. Sci. USA 96, 3507–3512 (1999).
Zarrinkar, P. P. & Sullenger, B. A. Optimizing the substrate specificity of a group I intron ribozyme. Biochemistry 38, 3426–3432 (1999).
Sullenger, B. A., Gallardo, H. F., Ungers, G. E. & Gilboa, E. Overexpression of TAR sequences renders cells resistant to human immunodeficiency virus replication. Cell 63, 601–608 (1990).
Lee, T. C., Sullenger, B. A., Gallardo, H. F., Ungers, G. E. & Gilboa, E. Overexpression of RRE-derived sequences inhibits HIV-1 replication in CEM cells. New Biol. 4, 66–74 (1992).
Kohn, D. B. et al. A clinical trial of retroviral-mediated transfer of a rev-responsive element decoy gene into CD34+ cells from the bone marrow of human immunodeficiency virus-1-infected children. Blood 94, 368–371 (1999).
Tuerk, C. & Gold, L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 249, 505–510 (1990).
Ellington, A. D. & Szostak, J. W. In vitro selection of RNA molecules that bind specific ligands. Nature 346, 818–822 (1990).
Gold, L., Polisky, B., Uhlenbeck, O. & Yarus, M. Diversity of oligonucleotide functions. Annu. Rev. Biochem. 64, 763–797 (1995).
White, R. R., Sullenger, B. A. & Rusconi, C. P. Developing aptamers into therapeutics. J. Clin. Invest. 106, 929–934 (2000).
Jellinek, D. et al. Potent 2′-amino-2′-deoxypyrimidine RNA inhibitors of basic fibroblast growth factor. Biochemistry 34, 11363–11372 (1995).
Ruckman, J. et al. 2′-Fluoropyrimidine RNA-based aptamers to the 165-amino acid form of vascular endothelial growth factor (VEGF165). Inhibition of receptor binding and VEGF-induced vascular permeability through interactions requiring the exon 7-encoded domain. J. Biol. Chem. 273, 20556–20567 (1998).
Hicke, B. J. & Stephens, A. W. Escort aptamers: a delivery service for diagnosis and therapy. J. Clin. Invest. 106, 923–928 (2000).
Bock, L. C., Griffin, L. C., Latham, J. A., Vermaas, E. H. & Toole, J. J. Selection of single-stranded DNA molecules that bind and inhibit human thrombin. Nature 355, 564–566 (1992).
Griffin, L. C., Tidmarsh, G. F., Bock, L. C., Toole, J. J. & Leung, L. L. In vivo anticoagulant properties of a novel nucleotide-based thrombin inhibitor and demonstration of regional anticoagulation in extracorporeal circuits. Blood 81, 3271–3276 (1993).
DeAnda, A. Jr et al. Pilot study of the efficacy of a thrombin inhibitor for use during cardiopulmonary bypass. Ann. Thoracic Surg. 58, 344–350 (1994).
Ostendorf, T. et al. Specific antagonism of PDGF prevents renal scarring in experimental glomerulonephritis. J. Am. Soc. Nephrol. 12, 909–918 (2001).
Drolet, D. W. et al. Pharmacokinetics and safety of an anti-vascular endothelial growth factor aptamer (NX1838) following injection into the vitreous humor of rhesus monkeys. Pharm. Res. 17, 1503–1510 (2000).
Yewdell, J. W., Norbury, C. C. & Bennink, J. R. Mechanisms of exogenous antigen presentation by MHC class I molecules in vitro and in vivo: implications for generating CD8+ T cell responses to infectious agents, tumors, transplants, and vaccines. Adv. Immunol. 73, 1–77 (1999).
Banchereau, J. & Steinman, R. M. Dendritic cells and the control of immunity. Nature 392, 245–252 (1998).
Boczkowski, D., Nair, S. K., Snyder, D. & Gilboa, E. Dendritic cells pulsed with RNA are potent antigen-presenting cells in vitro and in vivo. J. Exp. Med. 184, 465–472 (1996).
Koido, S. et al. Induction of antitumor immunity by vaccination of dendritic cells transfected with MUC1 RNA. J. Immunol. 165, 5713–5719 (2000).
Granstein, R. D., Ding, W. & Ozawa, H. Induction of anti-tumor immunity with epidermal cells pulsed with tumor-derived RNA or intradermal administration of RNA. J. Invest. Dermatol. 114, 632–636 (2000).
Zhang, W. et al. Enhanced therapeutic efficacy of tumor RNA-pulsed dendritic cells after genetic modification with lymphotactin. Hum. Gene Ther. 10, 1151–1161 (1999).
Boczkowski, D., Nair, S. K., Nam, J. H., Lyerly, H. K., & Gilboa, E. Induction of tumor immunity and cytotoxic T lymphocyte responses using dendritic cells transfected with messenger RNA amplified from tumor cells. Cancer Res. 60, 1028–1034 (2000).
Ashley, D. M. et al. Bone marrow-generated dendritic cells pulsed with tumor extracts or tumor RNA induce antitumor immunity against central nervous system tumors. J. Exp. Med. 186, 1177–1182 (1997).
Nair, S. K. et al. Induction of cytotoxic T cell responses and tumor immunity against unrelated tumors using telomerase reverse transcriptase RNA transfected dendritic cells. Nature Med. 6, 1011–1017 (2000)
Weissman, D. et al. HIV gag mRNA transfection of dendritic cells (DC) delivers encoded antigen to MHC class I and II molecules, causes DC maturation, and induces a potent human in vitro primary immune response. J. Immunol. 165, 4710–4717 (2000).
Van Tendeloo, V. et al. Highly efficient gene delivery by mRNA electroporation in human hematopoietic cells: superiority to lipofection and passive pulsing of mRNA and to electroporation of plasmid cDNA for tumor antigen loading of dendritic cells. Blood 98, 49–56 (2001).
Su, Z., Peluso, M. V., Raffegerst, S. H., Schendel, D. J. & Roskrow, M. A. The generation of LMP2a-specific cytotoxic T lymphocytes for the treatment of patients with Epstein-Barr virus-positive Hodgkin disease. Eur. J. Immunol. 31, 947–958 (2001).
Strobel, I. et al. Human dendritic cells transfected with either RNA or DNA encoding influenza matrix protein M1 differ in their ability to stimulate cytotoxic T lymphocytes. Gene Ther. 7, 2028–2035 (2000).
Saeboe-Larssen, S., Fossberg, E. & Gaudernack, G. mRNA-based electrotransfection of human dendritic cells and induction of cytotoxic T lymphocyte responses against the telomerase catalytic subunit (hTERT). J. Immunol. Meth. 259, 191–203 (2002).
Heiser, A. et al. Human dendritic cells transfected with renal tumor RNA stimulate polyclonal T-cell responses against antigens expressed by primary and metastatic tumors. Cancer Res. 61, 3388–3393 (2001).
Heiser, A. et al. Induction of polyclonal prostate cancer-specific CTL using dendritic cells transfected with amplified tumor RNA. J. Immunol. 166, 2953–2960 (2001).
Heiser, A. et al. Human dendritic cells transfected with RNA encoding prostate-specific antigen stimulate prostate-specific CTL responses in vitro. J. Immunol. 164, 5508–5514 (2000).
Thornburg, C., Boczkowski, D., Gilboa, E. & Nair, S. K. Induction of cytotoxic T lymphocytes with dendritic cells transfected with human papillomavirus E6 and E7 RNA: implications for cervical cancer immunotherapy. J. Immunother. 23, 412–418 (2000).
Nair, S. K. et al. Induction of primary carcinoembryonic antigen (CEA)-specific cytotoxic T lymphocytes in vitro using human dendritic cells transfected with RNA. Nature Biotechnol. 16, 364–369 (1998).
Srivastava, P. K. Do human cancers express shared protective antigens? Or the necessity of remembrance of things past. Semin. Immunol. 8, 295–302 (1996).
Gilboa, E. The makings of a tumor rejection antigen. Immunity 11, 263–270 (1999).
Heiser, A. et al. Autologous dendritic cells transfected with prostate-specific antigen RNA stimulate CTL responses against metastatic prostate tumors. J. Clin. Invest. 109, 409–417 (2002).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sullenger, B., Gilboa, E. Emerging clinical applications of RNA. Nature 418, 252–258 (2002). https://doi.org/10.1038/418252a
Issue Date:
DOI: https://doi.org/10.1038/418252a
This article is cited by
-
Self-assembly for hybrid biomaterial of uridine monophosphate to enhance the optical phenomena
Chemical Papers (2023)
-
Fluorescence Signal Amplification Strategies Based on DNA Nanotechnology for miRNA Detection
Chemical Research in Chinese Universities (2020)
-
Evolution of a General RNA-Cleaving FANA Enzyme
Nature Communications (2018)
-
The diversity of mtDNA rns introns among strains of Ophiostoma piliferum, Ophiostoma pluriannulatum and related species
SpringerPlus (2016)
-
Advances in time course extracellular production of human pre-miR-29b from Rhodovulum sulfidophilum
Applied Microbiology and Biotechnology (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.