Abstract
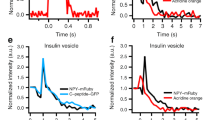
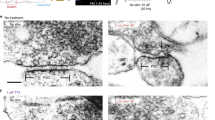
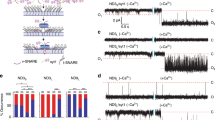
The vesicles that package neurotransmitters fall into two distinct classes, large dense-core vesicles (LDCVs) and small synaptic vesicles, the coexistence of which is widespread in nerve terminals1. High resolution capacitance recording reveals unitary steps proportional to vesicle size. Measurements of capacitance steps during LDCV and secretory granule fusion in endocrine and immune cells have provided important insights into exocytosis2,3,4; however, extending these measurements to small synaptic vesicles has proven difficult. Here we report single vesicle capacitance steps in posterior pituitary nerve terminals. These nerve terminals contain neuropeptide-laden LDCVs, as well as microvesicles. Microvesicles are similar to synaptic vesicles in size, morphology5 and molecular composition6,7,8, but their contents are unknown. Capacitance steps of two characteristic sizes, corresponding with microvesicles and LDCVs, were detected in patches of nerve terminal membrane. Both types of vesicles fuse in response to depolarization-induced Ca2+ entry. Both undergo a reversible fusion process commonly referred to as ‘kiss-and-run’, but only rarely. Fusion pores seen during microvesicle kiss-and-run have a conductance of 19 pS, 11 times smaller than LDCV fusion pores. Thus, LDCVs and microvesicles use structurally different intermediates during exocytosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
De Camilli, P. & Jahn, R. Pathways to regulated exocytosis in neurons. Ann. Rev. Physiol. 52, 625–645 (1990)
Neher, E. & Marty, A. Discrete changes of cell membrane capacitance observed under conditions of enhanced secretion in bovine adrenal chromaffin cells. Proc. Natl Acad. Sci. USA 79, 6712–6716 (1982)
Fernandez, J. M., Neher, E. & Gomperts, B. D. Capacitance measurements reveal stepwise fusion events in degranulating mast cells. Nature 312, 453–455 (1984)
Lollike, K., Borregaard, N. & Lindau, M. The exocytotic fusion pore of small granules has a conductance similar to an ion channel. J. Cell Biol. 129, 99–104 (1995)
Morris, J. F., Nordmann, J. J. & Dyball, R. E. J. Structure-function correlation in mammalian neurosecretion. Int. Rev. Exp. Pathol. 18, 1–95 (1978)
Navone, F. et al. Microvesicles of the neurohypophysis are biochemically related to small synaptic vesicles of presynaptic nerve terminals. J. Cell Biol. 109, 3425–3433 (1989)
Walch-Solimena, C. et al. Synaptotagmin: a membrane constituent of neuropeptide-containing large dense-core vesicles. J. Neurosci. 13, 3895–3903 (1993)
Pupier, S. et al. Cysteine string proteins associated with secretory granules of the rat neurohypophysis. J. Neurosci. 17, 2722–2727 (1997)
Debus, K. & Lindau, M. Resolution of patch capacitance recordings and of fusion pore conductance in small vesicles. Biophys. J. 78, 2983–2997 (2000)
Gentet, L. J., Stuart, G. J. & Clements, J. D. Direct measurement of specific membrane capacitance in neurons. Biophys. J. 79, 314–320 (2000)
Nagasawa, J., Douglas, W. W. & Schulz, R. A. Micropinocytotic origin of coated and smooth microvesicles (‘synaptic vesicles’) in neurosecretory terminals of posterior pituitary glands demonstrated by incorporation of horse-radish peroxidase. Nature 332, 341–342 (1971)
Morris, J. F. & Nordmann, J. J. Membrane recapture after hormone release from nerve endings in the neural lobe of the rat pituitary gland. Neuroscience 5, 639–649 (1980)
Hsu, S.-F. & Jackson, M. B. Rapid exocytosis and endocytosis in nerve terminal of the rat posterior pituitary. J. Physiol. 494.2, 539–553 (1996)
Rosenboom, H. & Lindau, M. Exo-endocytosis and closing of the fission pore during endocytosis in single pituitary nerve terminals internally perfused with high calcium concentrations. Proc. Natl Acad. Sci. USA 91, 5267–5271 (1994)
Lindau, M. & Almers, W. Structure and function of fusion pores in exocytosis and ectoplasmic membrane fusion. Curr. Opin. Neurobiol. 7, 509–517 (1995)
Fesce, R., Grohovaz, F., Valtorta, F. & Meldolesi, J. Neurotransmitter release: fusion or ‘kiss-and-run’? Trends Cell Biol. 4, 1–4 (1994)
Tse, F. W., Iwata, A. & Almers, W. Membrane flux through the pore formed by a fusogenic viral envelope protein during cell fusion. J. Cell Biol. 121, 543–552 (1993)
Zimmerberg, J., Blumenthal, R., Curran, M., Sarker, D. & Morris, S. Restricted movement of lipid and aqueous dyes through pores formed by influenza hemagglutinin during cell fusion. J. Cell Biol. 127, 1885–1894 (1994)
Breckenridge, L. J. & Almers, W. Currents through the fusion pore that forms during exocytosis of a secretory granule. Nature 328, 814–817 (1987)
Spruce, A. E., Breckenridge, L. J., Lee, A. K. & Almers, W. Properties of the fusion pore that forms during exocytosis of a mast cell secretory granule. Neuron 4, 643–654 (1990)
Almers, W. et al. Millisecond studies of single membrane fusion events. Ann. NY Acad. Sci. 635, 318–327 (1991)
Chow, R. H., von Rüden, L. & Neher, E. Delay in vesicle fusion revealed by electrochemical monitoring of single secretory events in adrenal chromaffin cells. Nature 356, 60–63 (1992)
Bruns, D. & Jahn, R. Real-time measurement of transmitter release from single synaptic vesicles. Nature 377, 62–65 (1995)
Stiles, J. R., Van Helden, D., Bartol, T. M., Salpeter, E. E. & Salpeter, M. M. Miniature endplate current rise times < 100 μs from improved dual recordings can be modeled with passive acetylcholine diffusion from a synaptic vesicle. Proc. Natl Acad. Sci. USA 93, 5747–5752 (1996)
Khanin, R., Parnas, H. & Segel, L. Diffusion cannot govern the discharge of neurotransmitter in fast synapses. Biophys. J. 67, 966–972 (1994)
Khanin, R., Parnas, H. & Segel, L. A mechanism for discharge of charged excitatory neurotransmitter. Biophys. J. 72, 507–521 (1997)
Nanavati, C. & Fernandez, J. M. The secretory granule matrix: a fast acting smart polymer. Science 259, 963–965 (1993)
Ales, E. et al. High calcium concentrations shift the mode of exocytosis to the kiss-and-run mechanism. Nature Cell Biol. 1, 40–44 (1999)
Verhage, M. et al. Differential release of amino acids, neuropeptides, and catecholamines from isolated nerve terminals. Neuron 6, 517–524 (1991)
Rae, J. L. & Levis, R. A. A method for exceptionally low noise single channel recordings. Pflügers Arch. Eur. J. Physiol. 420, 618–620 (1992)
Acknowledgements
We thank M. Lindau for advice on capacitance measurements, D.L. Armstrong for suggesting the use of oil to reduce noise, and E.R. Chapman, C.-T. Wang, P.Y. Chang, G.P. Ahern and X. Han for helpful comments on the manuscript. We thank R. J. Massey of the UW Electron Microscope Facility for performing electron microscopy. This research was supported by a grant from NIH and a predoctoral fellowship from the AHA to V.A.K.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Klyachko, V., Jackson, M. Capacitance steps and fusion pores of small and large-dense-core vesicles in nerve terminals. Nature 418, 89–92 (2002). https://doi.org/10.1038/nature00852
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature00852
This article is cited by
-
Membrane transformations of fusion and budding
Nature Communications (2024)
-
Exploring the Therapeutic Effect of Neurotrophins and Neuropeptides in Neurodegenerative Diseases: at a Glance
Molecular Neurobiology (2023)
-
Various approaches for measurement of synaptic vesicle endocytosis at the central nerve terminal
Archives of Pharmacal Research (2019)
-
Dynamics and number of trans-SNARE complexes determine nascent fusion pore properties
Nature (2018)
-
VGLUT1 functions as a glutamate/proton exchanger with chloride channel activity in hippocampal glutamatergic synapses
Nature Communications (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.